Compositions for removing stains from dental surfaces, and methods of making and using the same
a technology for dental surfaces and compositions, applied in the field of stain removal compositions, can solve the problems of inability to clean teeth mechanically, intrinsic tooth staining discoloration or stained from chromogenic, and difficulty in addressing intrinsic tooth staining, etc., to achieve synergistic stain removal or tooth whitening effect, improve stain removal efficacy, and reduce the effective amount of each component incorporated
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Experimental Results from a Stain Removal Study
Materials and Methods
[0074]A flow system was prepared to treat hydroxyapatite (HAP) disks for staining. Tea, coffee and porcine gastric mucin solution was mixed to yield a staining broth. The staining broth was circulated through the flow system in contact with the HAP disks at a rate of 15 ml per minute for about 96 hours at 37° C. The resulting stained HAP disks were rinsed in a solution of artificial saliva at a pH of about 7 and allowed to dry for about 2 hours at room temperature.
[0075]Once the HAP disks were dry, a baseline stain reading was determined for each HAP disk by measuring its diffuse reflectance absorbance value using a Minolta spectrometer. The measurement was made over the entire visible color spectrum in accordance with the Commission International de L'Eclairage Laboratory (CIELAB) color scale. The CIELAB color scale quantifies color according to 3 parameters: lightness-darkness scale (L), red-green chroma (RGC), an...
example 2
In-Vitro Stain Removal Study
Materials and Methods
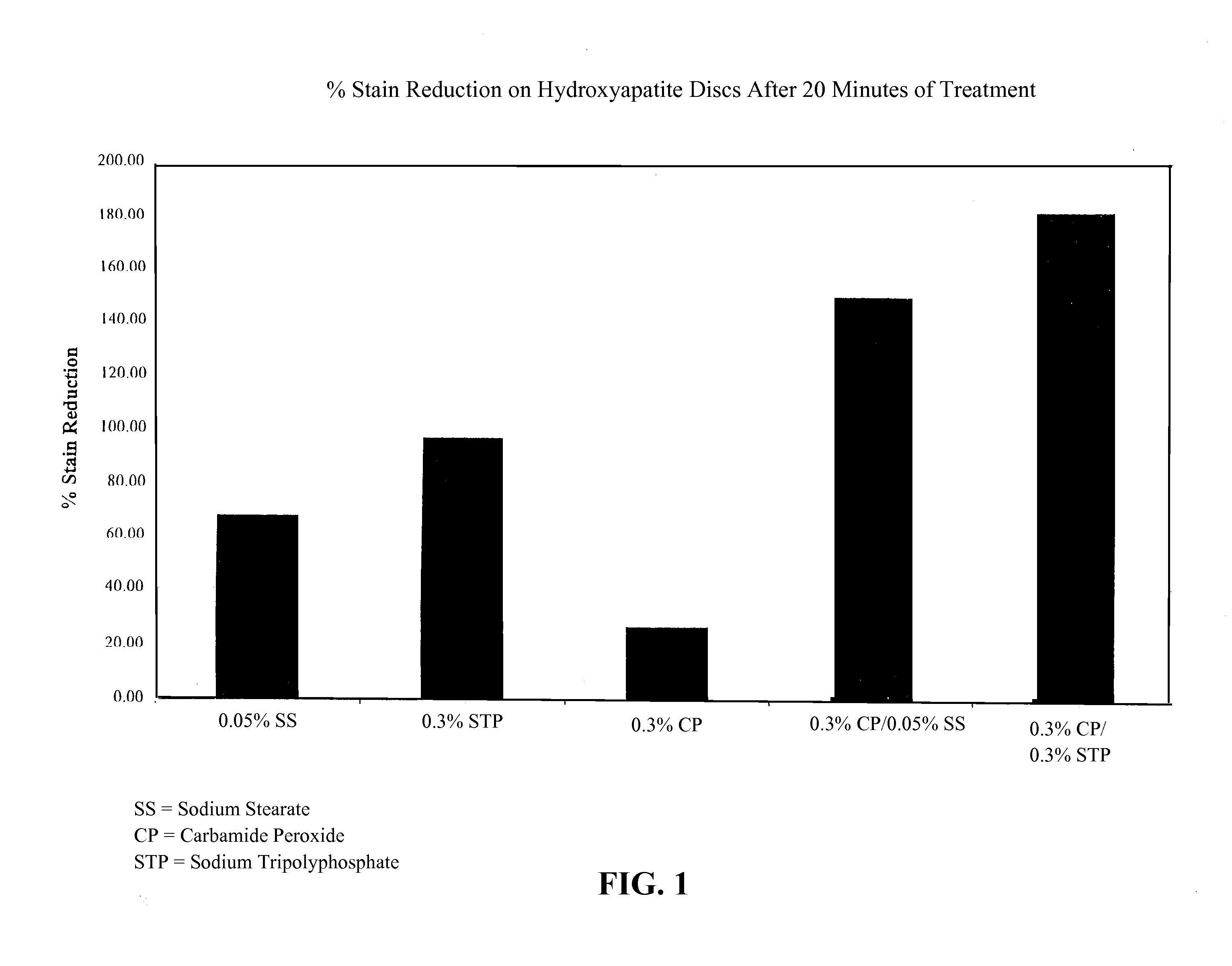
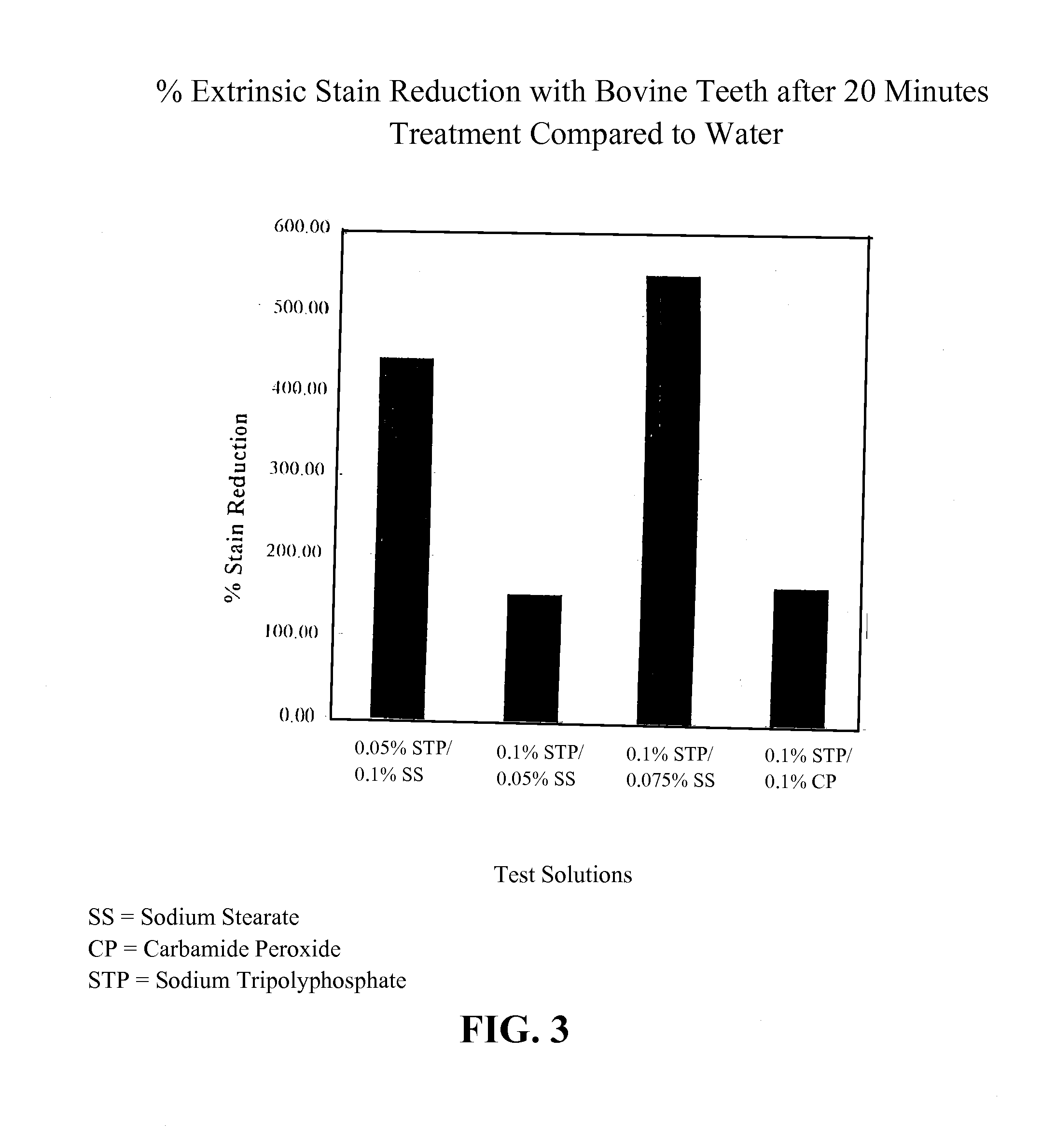
[0079]Several hydroxyapatite disks were prepared and pretreated to form on the surface of the disks a biofilm that was discolored with a vegetable stain. The color intensity of each disk was then determined utilizing a Chrom-A-Meter. The disks were suspended in either water or a test solution containing a solution selected from 1) 0.05% sodium stearate; 2) 0.3% sodium tripolyphosphate, 3) 0.3% carbamide peroxide; 4) 0.3% carbamide peroxide and 0.05% sodium stearate; and 5) 0.3% carbamide peroxide and 0.3% sodium tripolyphosphate, respectively. Water acts as a control to take into account the differences in the disk composition and the variation in the stain thickness between batches. The disks were treated for two ten (10) minute intervals, and characterized by the Chrom-A-Meter after each treatment interval. The change in color is expressed by ΔE values relative to the water treatment alone. The percent stain reduction is then calcul...
example 3
In-Vitro Stain Removal Study
Materials and Methods
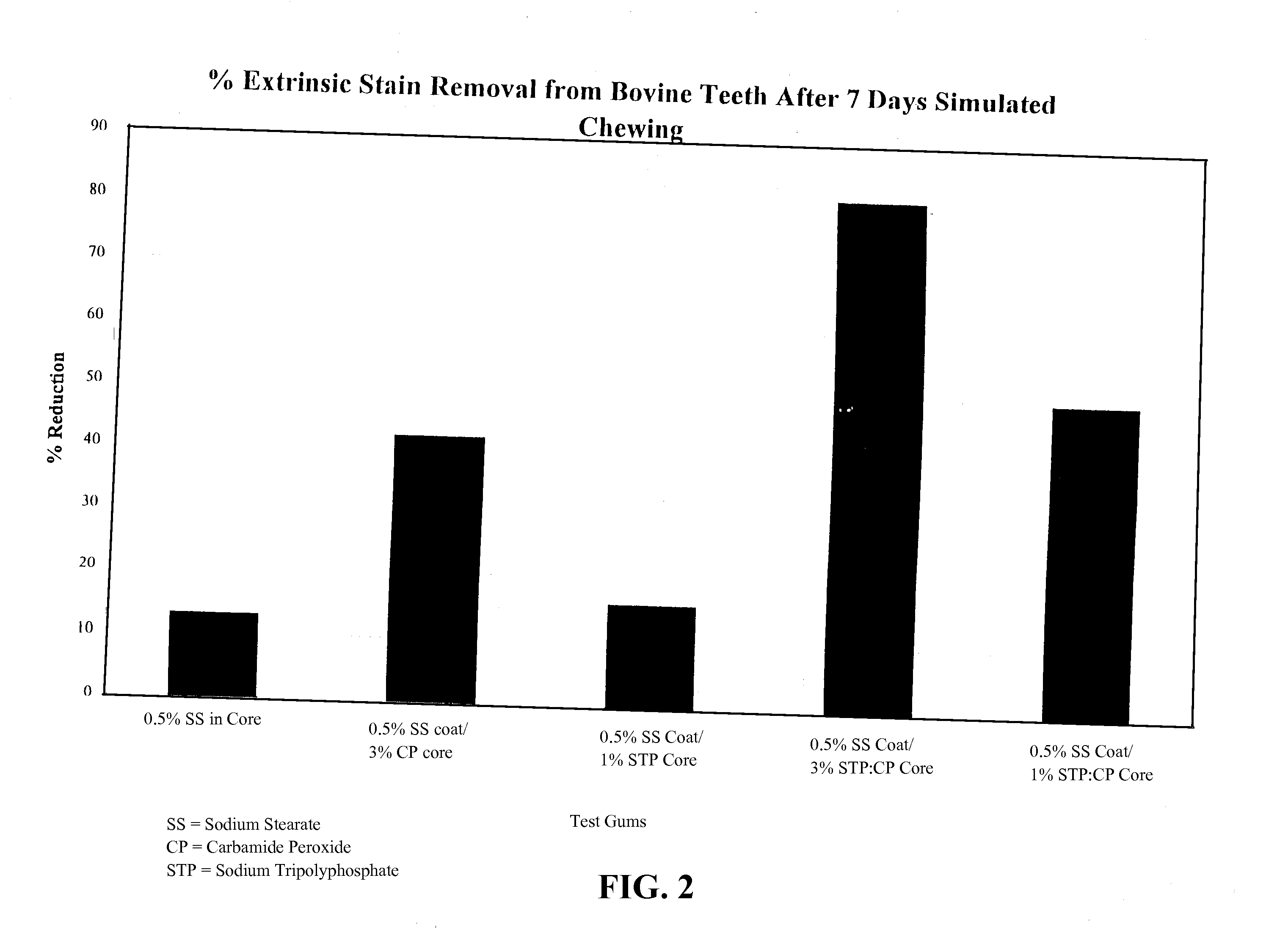
[0085]Five coated gum samples were tested in a stain removal test model each an active compositions selected from 1) 0.5% sodium stearate in a core portion; 2) 0.5% sodium stearate in a coating portion and 3% carbamide peroxide in a core portion; 3) 0.5% sodium stearate in a coating portion and 1% sodium tripolyphosphate in a core portion; 4) 0.5% sodium stearate in a coating portion and 3% sodium tripolyphosphate / carbamide peroxide in a core portion; and 5) 0.5% sodium stearate in a coating portion and 1% sodium tripolyphosphate / carbamide peroxide in a core portion. The gum samples were masticated by a chewing machine, which was outfitted with stained bovine teeth providing chewing surfaces to simulate the top and bottom teeth in a human mouth. The samples were chewed for five minutes. The bovine teeth were checked with a Chrom-A-Meter before they were outfitted into the machine, and checked again after simulated chewing over a one w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mesh size | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com