Control of hard water scaling in electrochemical cells
a technology of hard water scaling and electrochemical cells, applied in the field of water electrolysis, can solve the problems of increasing voltage demand, affecting the efficiency and convenience of electrolysis systems, and affecting the efficiency of electrolysis systems, and achieve the effect of effective cleaning and/or sanitizing surfaces
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Electrode Scaling in Hard Water. A clean 1″×6″ pair of ruthenium (Ru) coated DSA electrodes for hypochlorite production was analyzed for hard water scaling. The pair of electrodes were placed in a stirred 4 L solution of 1000 ppm of sodium chloride. The sodium chloride solution was made using 17 grain (gpg) hard water. The electrodes in the sodium chloride solution were energized with about 8-12 volts / 0.5 amps from a DC power source. After 4 hours in the electrochemical cell of the sodium chloride solution, the electrodes were removed and air dried. The electrodes were visually examined, revealing a heavy white scale covering the inside of the cathode.
example 2
Prevention of Electrode Scaling in Hard Water With Threshold Agent. The same methods from Example 1 were repeated using a variety of polyacrylate threshold agents and a known reverse EO-PO copolymer threshold agent. The threshold agents were added to the 17 grain hard water sodium chloride solution.
First, using a clean 1″×6″ pair of Ru-coated DSA electrodes for hypochlorite production. The electrodes were placed in a stirred 4 L solution of 1000 ppm of sodium chloride and 100 ppm Acumer 1000 (an acrylic homopolymer of about 2000 molecular weight, available from Rohm & Haas as a 48% solids product). The electrodes in the sodium chloride solution were energized with about 8-12 volts / 0.5 amps from a DC power source. After 4 hours, the electrodes were removed and air dried. No build-up of hard water scale was observed on the cathode submerged in the electrochemical cell containing the threshold agent.
The methods were repeated using solutions containing alternative threshold agents. Elec...
example 3
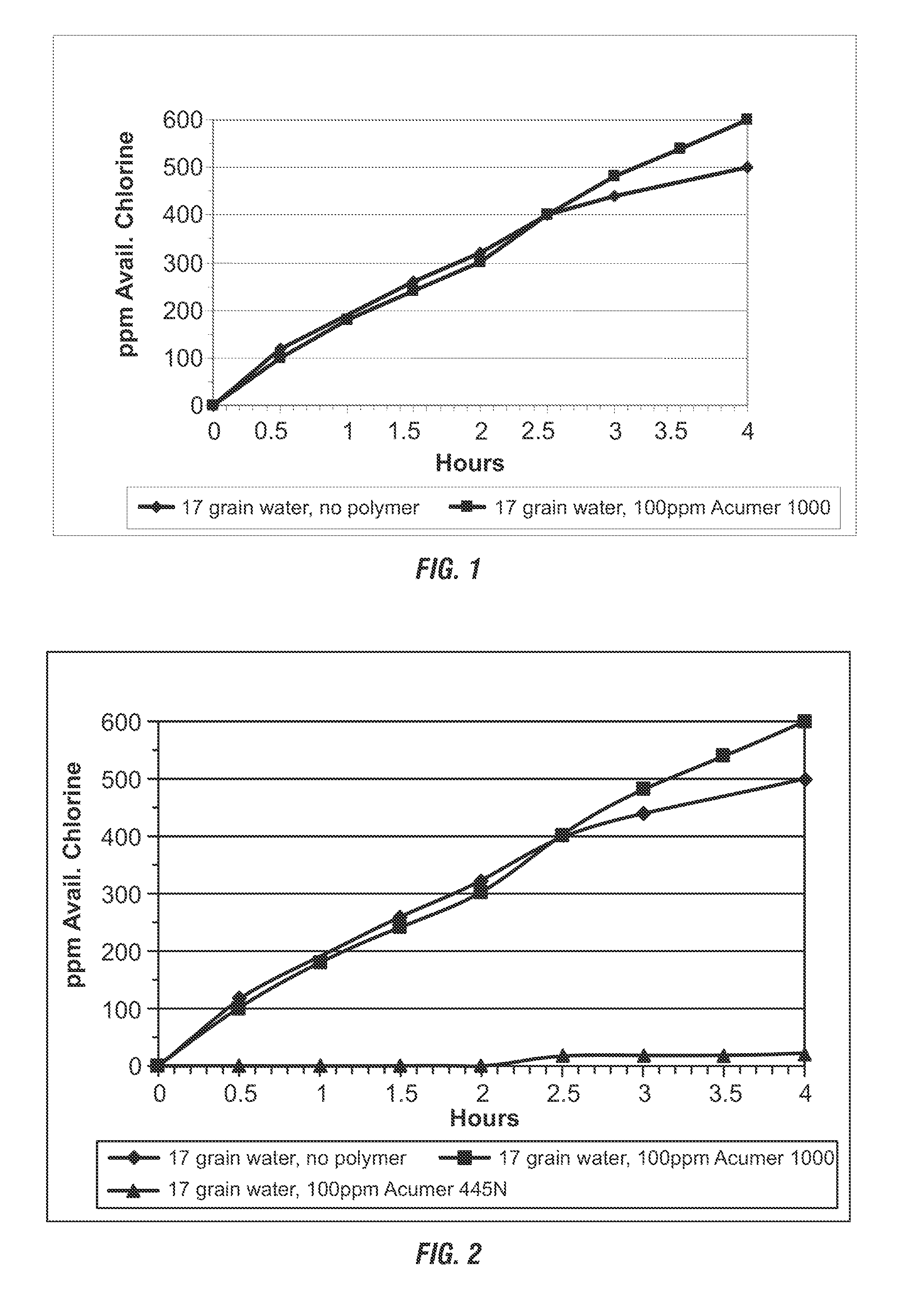
Effect of Threshold Inhibiter on Hypochlorite Formation Rate. A stirred 4 L solution of 1000 ppm of sodium chloride and 100 ppm Acumer 1000 (a polyacrylate of about 2000 molecular weight available from Akzo) was made using 17 grain hard water. A clean 1″×6″ pair of Ru-coated DSA electrodes for hypochlorite production were placed in the solution. The electrodes were energized with about 8-12 volts / 0.5 amps from a DC power source in the electrochemical cell. Samples were periodically removed from the solution and titrated for available chlorine. FIG. 1 shows a plot of the titration data versus time, demonstrating that the threshold agent Acumer 1000 did not result in any decrease in the rate of hypochlorite formation in the electrochemical cell. Therefore, the polyacrylate threshold agent is capable of preventing hard water scale formation on the cathodes of the electrolyzed cell without decreasing the product of hypochlorite.
The methods were repeated using the same threshold agent (A...
PUM
| Property | Measurement | Unit |
|---|---|---|
| threshold | aaaaa | aaaaa |
| threshold | aaaaa | aaaaa |
| threshold | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com