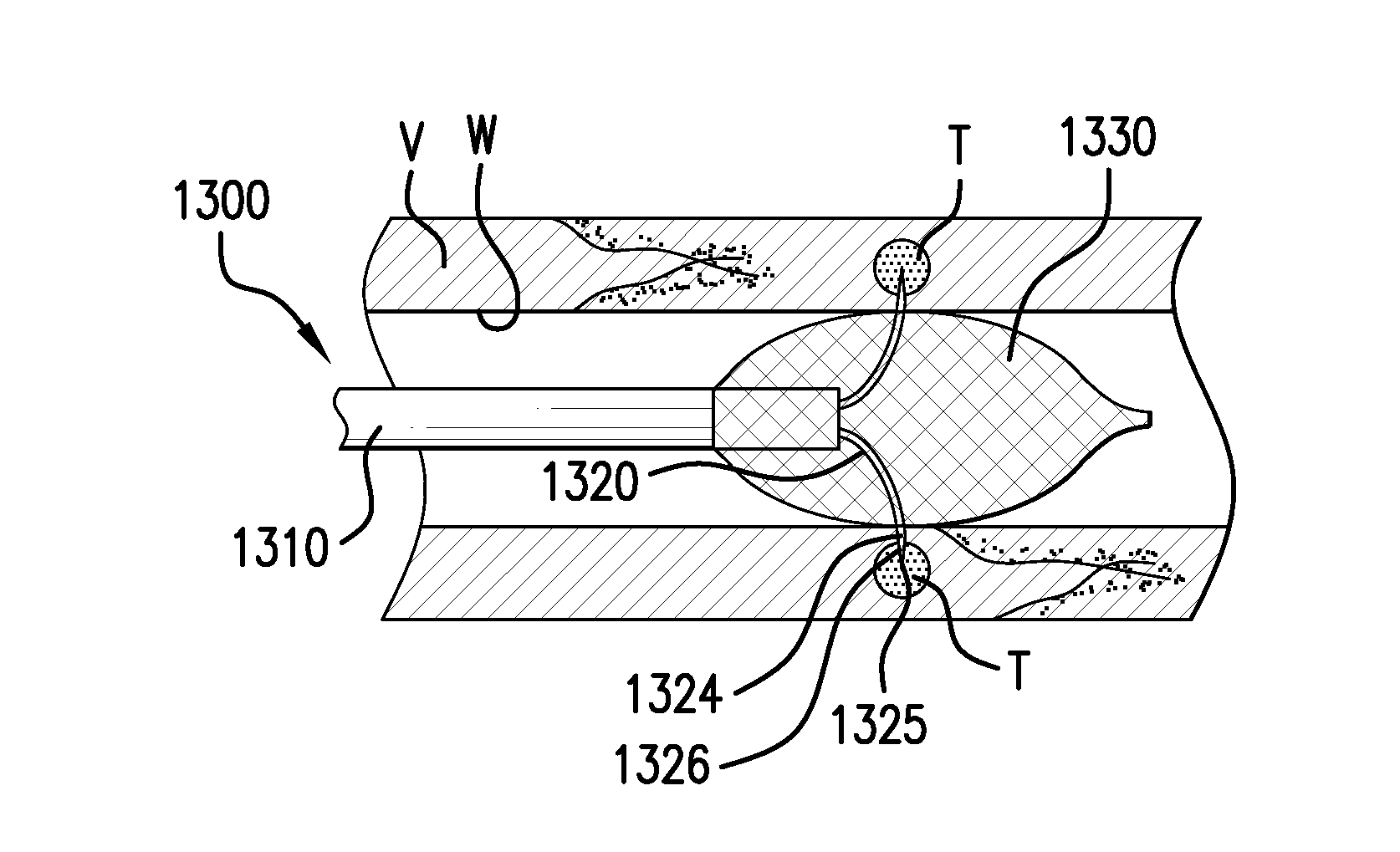
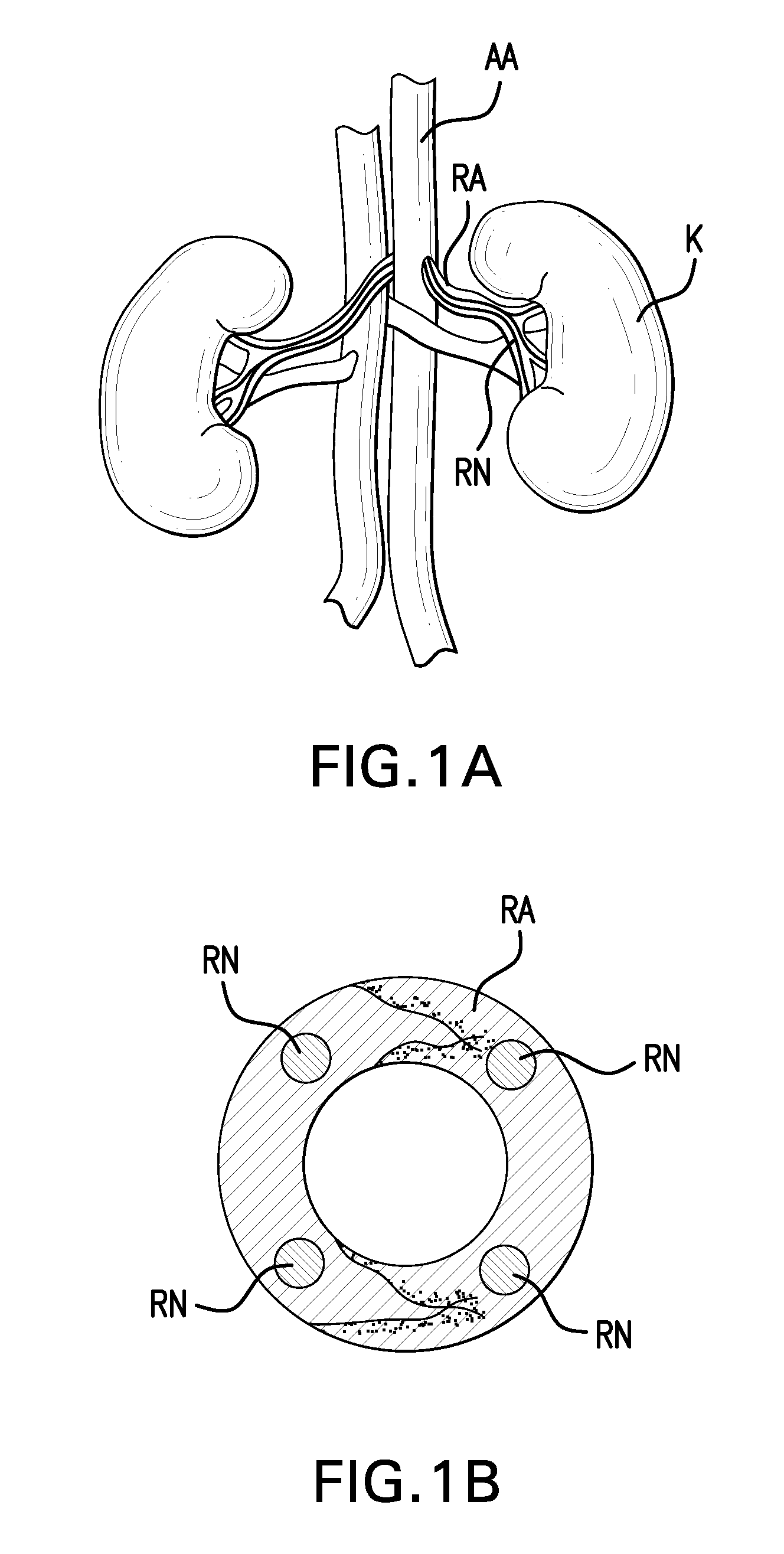
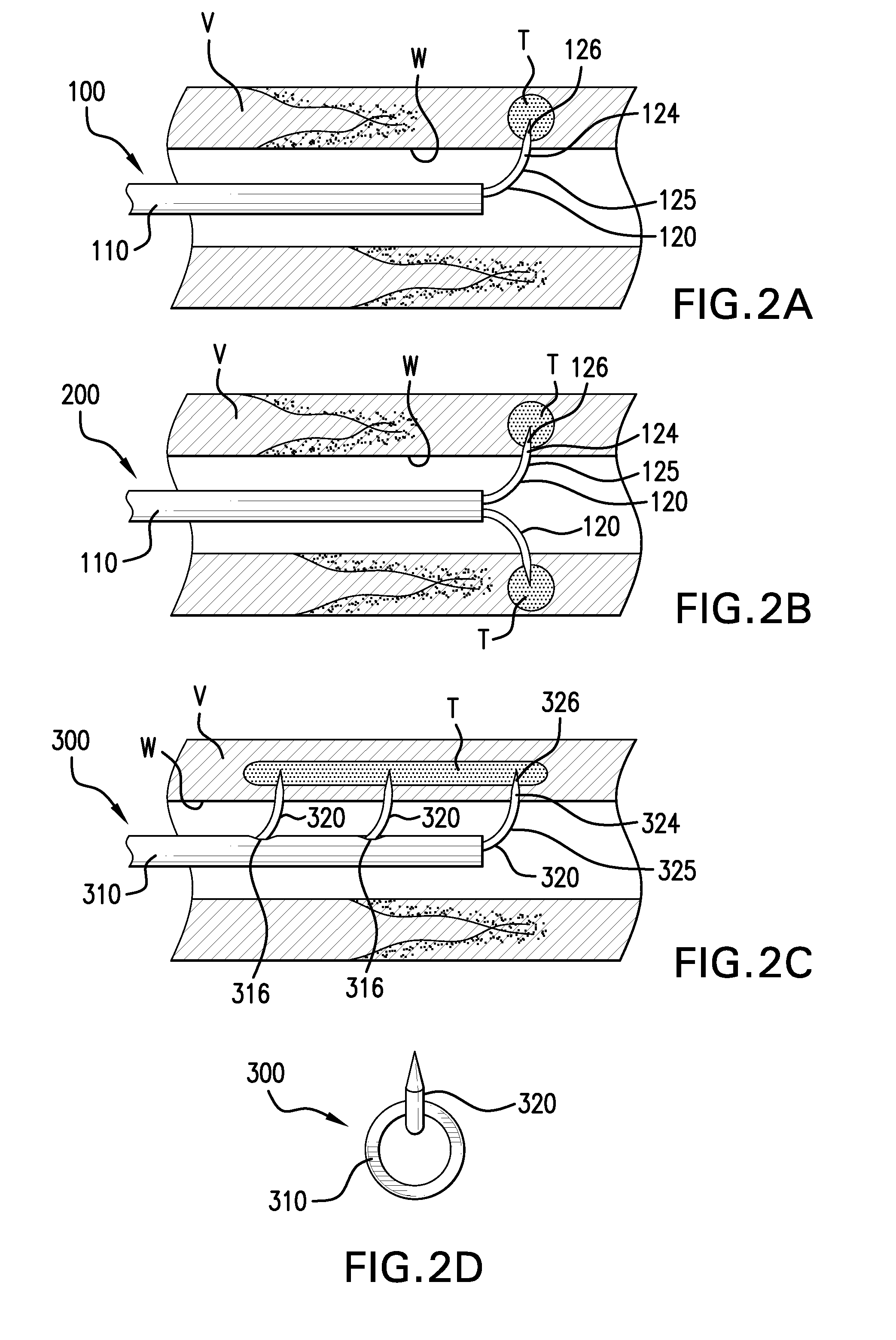
Methods and devices for denervation
a technology of denervation and denervation tube, which is applied in the field of denervation tube methods and devices, can solve the problems of limiting the selection of particular therapeutic agents, difficult to deliver therapeutic agents with sufficient precision to the renal nerve, and toxic to the patient or surrounding tissues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
A vasoconstrictor (antidiuretic hormone (ADH or vasopressin) or tetrahydrozoline) is first administered to vessels surrounding the target site to minimize leakage of the priming or secondary agents.
A priming agent of digoxin at a concentration of 0.0001-10 mM in a volume of 0.05-2 cc is then administered at a nerve proximal site to prime the neurons by inhibiting the transport of potassium and sodium across the nerve cell membrane and subsequently inducing an intracellular calcium flux.
Approximately 0.1-20 minutes later, a secondary agent of glutamate at a concentration of 0.1-700 mM in a volume of 0.05-2 cc is then administered at a nerve proximal site to induce neuronal excitotoxicity.
Vasoconstriction results from the increased concentration of calcium (Ca2+ ions) within vascular smooth muscle cells. However, the specific mechanisms for generating an increased intracellular concentration of calcium depends on the vasoconstrictor. Two common stimuli for eliciting smooth muscle cont...
example 2
A vasoconstrictor (antidiuretic hormone (ADH or vasopressin) or tetrahydrozoline) is first administered to vessels surrounding the target site to minimize leakage of the priming or secondary agents.
A priming agent of proscillaridin at a concentration of 0.0001-10 mM in a volume of 0.05-2 cc is then administered at a nerve proximal site to prime the neurons by inhibiting the transport of potassium and sodium across the nerve cell membrane and subsequently inducing an intracellular calcium flux.
Approximately 0.1-20 minutes later, a secondary agent of domoic acid at a concentration of 0.00005-0.005 mM in a volume of 0.05-2 cc is then administered at a nerve proximal site to induce neuronal excitotoxicity.
Proscillaridin binds to a site on the extracellular aspect of the α-subunit of the Na+ / K+ ATPase pump in the membranes of heart cells (myocytes) and decreases its function. This causes an increase in the level of sodium ions in the myocytes, which leads to a rise in the level of intrac...
example 3
A vasoconstrictor (antidiuretic hormone (ADH or vasopressin) or tetrahydrozoline) is first administered to vessels surrounding the target site to minimize leakage of the priming or secondary agents.
A priming agent of N-Methyl-D-aspartic acid (NMDA) at a concentration of 0.01-300 mM in a volume of 0.05-2 cc is then administered at a nerve proximal site to prime the neurons by inducing excitatory intracellular signaling.
Approximately 0.1-20 minutes later, a secondary agent of digoxin at a concentration of 0.0001-10 mM in a volume of 0.05-2 cc is then administered at a nerve proximal site to inhibit the transport of potassium and sodium across the nerve cell membrane and subsequently induce high levels of intracellular calcium to mediate pro-apoptotic signaling and neuronal toxicity.
N-Methyl-D-aspartic acid (NMDA) is an amino acid derivative, which acts as a specific agonist at the NMDA receptor mimicking the action of glutamate, the neurotransmitter, which normally acts at that recept...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar mass | aaaaa | aaaaa |
| Molar mass | aaaaa | aaaaa |
| Molar mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com