Immunotherapy targeting intracellular pathogens
a technology of immunotherapy and intracellular pathogens, applied in the direction of carrier-bound antigen/hapten ingredients, peptide sources, carrier-antigen complex architecture, etc., can solve the problems of limited vaccine effectiveness and severe affecting the intracellular survival of bacterial pathogens by tryptophan deprivation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Cytotoxic Regulatory T Cells to Adenovirus Elicit Increased Generation of Reactive Oxygen Intermediates in Dendritic Cells Presenting the Cognate Peptide
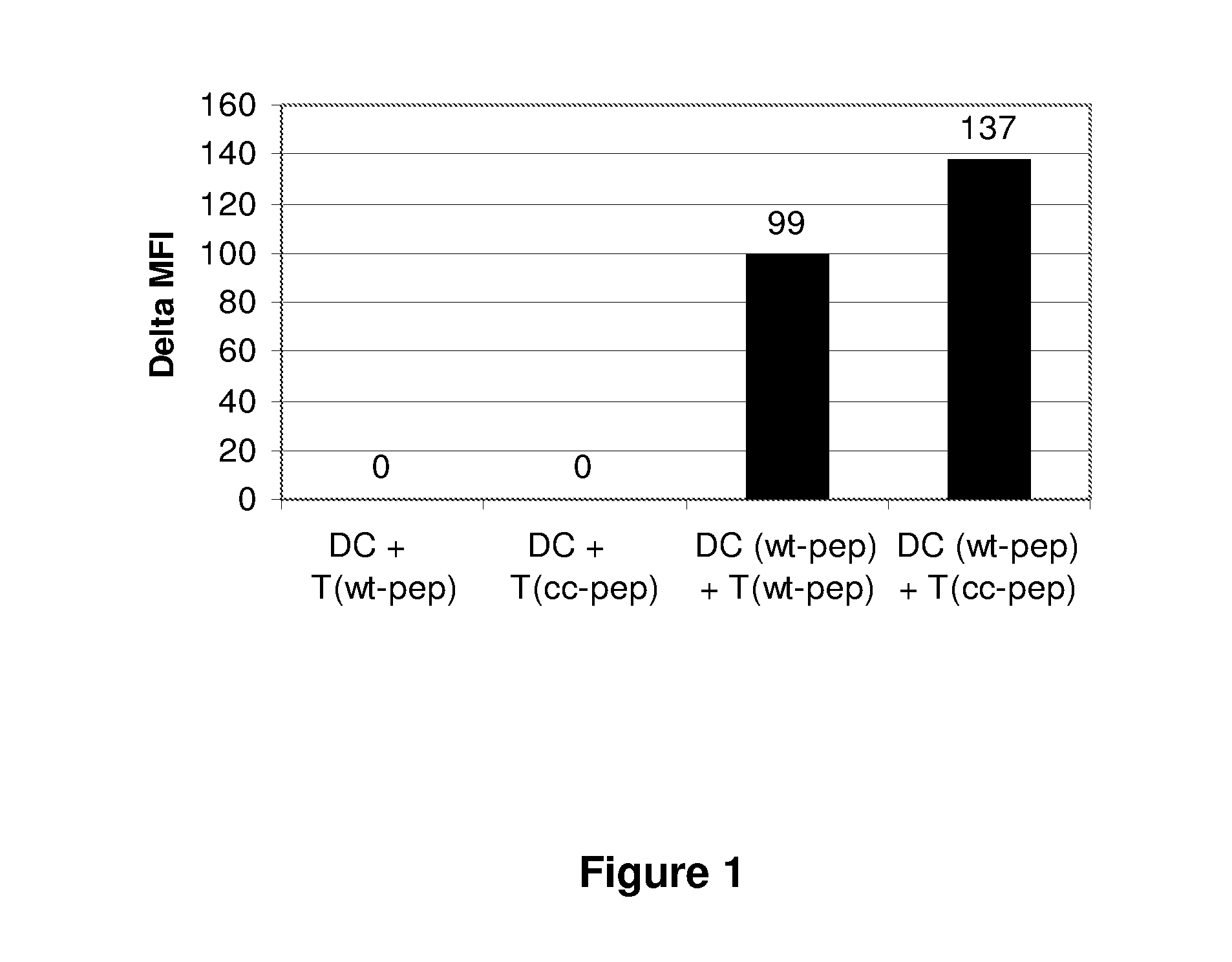
[0100]Adenovirus of serotype 5 (Ad.RR5, E1 / E3-deleted) was used in these experiments. Thus, 5 μL of a solution containing 2×1011 viral particles / ml was administered by the intravenous route to 6 weeks old C57BI / 6 mice. Ten days later, the spleen of such mice was recovered and CD4+ T cells purified by magnetic sorting.
[0101]A T cell epitope was identified within the sequence of the major capsid protein, by a combination of algorithms. A T cell epitope encompassing amino acid residues 912-921 was selected, with sequence: PTLLYVLFEV (SEQ ID NO:1; natural epitope). A synthetic peptide encoding this natural epitope sequence was obtained. A second peptide additionally containing a thioreductase consensus sequence (or redox motif) was synthesised and has the sequence: CHGCPTLLYVLFEV (SEQ ID NO:2; redox motif underlined; modified epitope).
[...
example 2
[0105]Mycobacterium tuberculosis is responsible for thousands of deaths every year. The only available vaccination, the Calmette-Guérin mycobacterium bovis-based vaccine (BCG), is not efficient. In addition, several mycobacterium strains show resistance to conventional chemotherapy. Antigen-specific CD4+ cells are known to occur in tuberculosis (Winslow et al. (2003) J. Immunol. 170:2046-2052), which can be protective (Khader et al. (2007) Nature Immunol. 8:369-377).
[0106]The early secretory antigen (ESA) produced by M. tuberculosis is one of the main antigens recognised both by humans and animals such as mice. A dominant T cell epitope, called ESAT-6, corresponding to the amino acid sequence 1-20, has been mapped and shown to be promiscuous, as it activates CD4+ T cells in major mouse strains and in humans. A (natural) T-cell epitope has been identified that contains amino acids 3-17, with sequence: FAGIEAAAS (SEQ ID NO:3). Addition of a consensus sequenc...
example 3
[0112]Plasmodium falciparum is a parasite responsible for malaria. The disease develops after mosquito biting. Infected mosquitoes inject sporozoites from Plasmodium into the bloodstream, which are taken by hepatocytes in which they transform into the merozoite form. Upon lysis of hepatocytes merozoites are taken up by erythrocytes, which results in massive hemolysis.
[0113]There is no satisfying vaccine available and malaria is responsible for ±1 million deaths a year, despite the use of chemicals to which the parasite often becomes resistant, such as nivaquine.
[0114]There are two steps at which vaccination could be envisaged (De Groot et al. (1989) J. Immunol. 142, 4000-4005; Reece et al. (2004) Nature Med. 10, 406-410). Prevention could be best obtained if the sporozoite form of the parasite was prevented from invading hepatocytes. Attenuation of ongoing disease could be achieved by targeting the merozoite form to prevent erythrocyte entry. Current efforts in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| molecular mass | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com