Electrode configuration of electrolysers to protect catalyst from oxidation
a technology of electrolyte and catalyst, which is applied in the field of electrolyte, can solve the problems of increasing cell voltage, increasing electrochemical losses, and current electrolyte technology is not mature enough for dynamic and intermittent operation, and achieves the effect of preventing the oxidation of anodic active catalyst materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
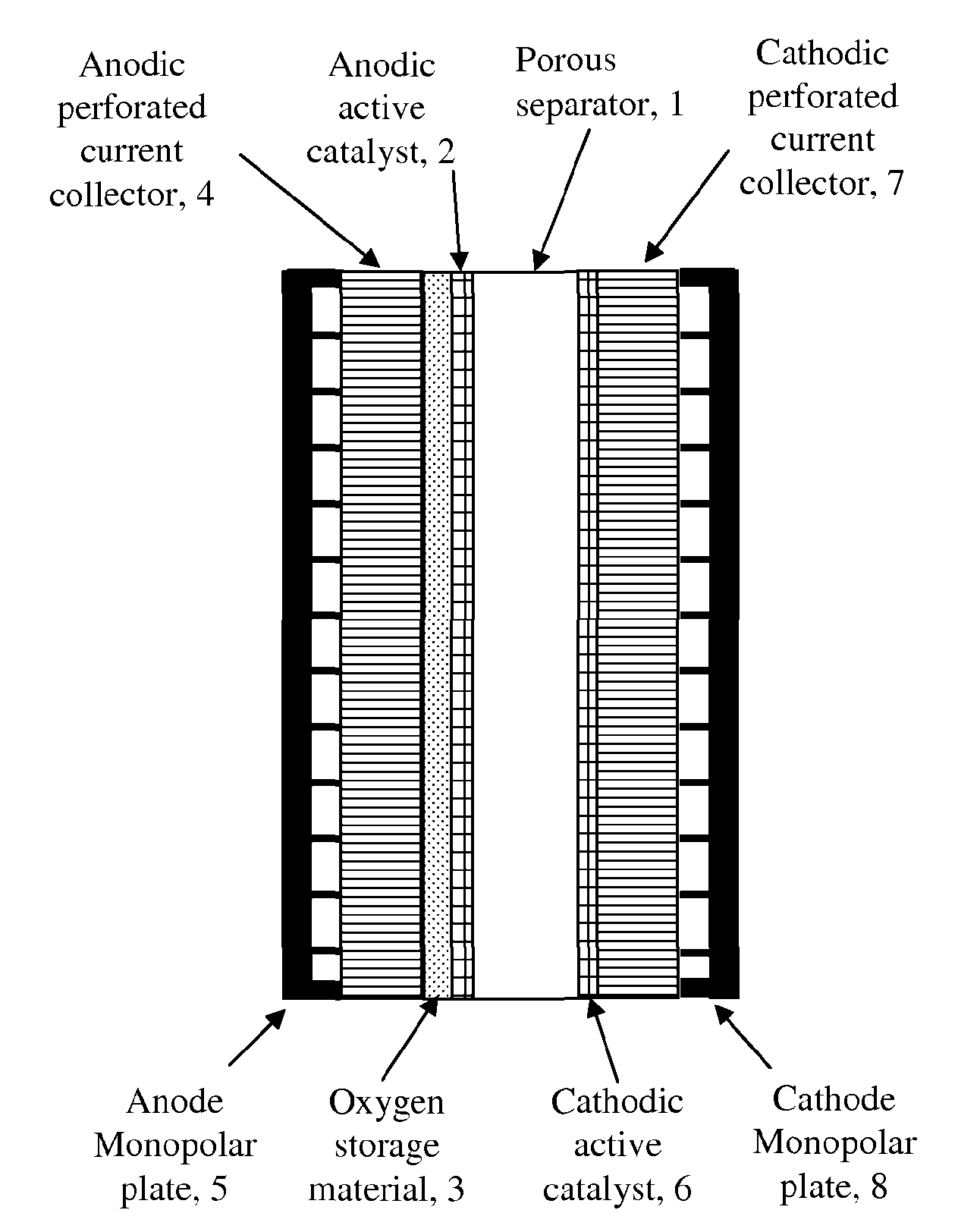
[0066]FIG. 1 shows the first example of one of the various electrode configurations according to an embodiment of the invention.
[0067]As shown in FIG. 1, oxygen storage material 3 is deposited onto an anodic perforated current collector 4 by various methods for example spraying, screen printing, hot pressing, sintering, thermal spraying, electroplating, electroforming, co-deposition by electroplating, electroless plating, dip coating, painting etc. The anodic active catalyst 2 is then deposited on top of the oxygen storage material 3 by various methods for example spraying, screen printing, hot pressing, sintering, thermal spraying, electroplating, electroforming, co-deposition by electroplating, electroless plating, dip coating, painting etc. The complete anode structure is then placed on one side of a porous separator 1.
[0068]An anode monopolar plate 5 is compressed to the back of the anodic perforated current collector 4 for supplying electricity and taking oxygen gas out of the ...
example 2
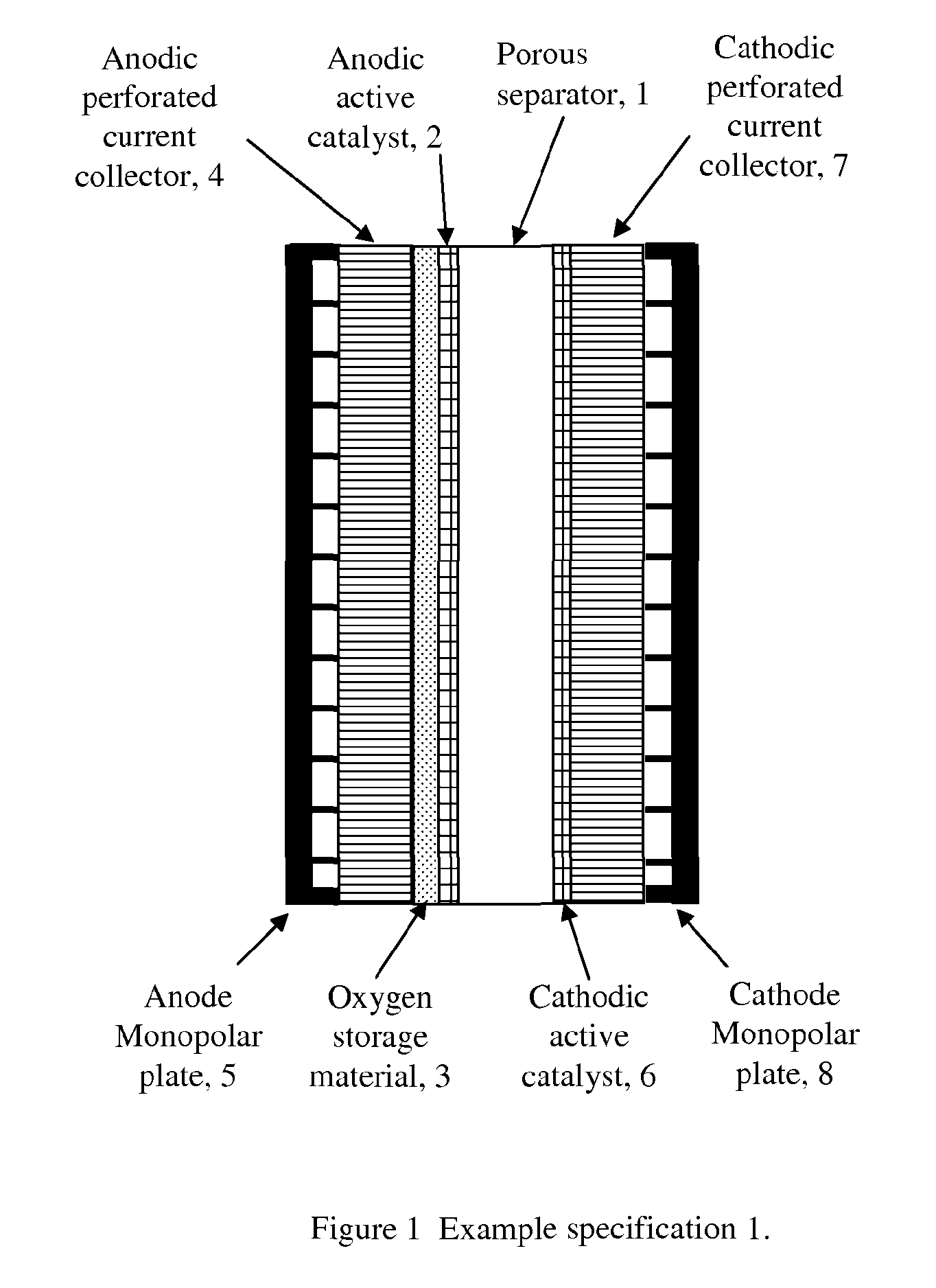
[0070]FIG. 2 shows the second example of one of the various electrode configurations according to an embodiment of the invention.
[0071]As shown in FIG. 2, an anodic active catalyst 2 is deposited onto an anodic perforated current collector 4 by various methods for example spraying, screen printing, hot pressing, sintering, thermal spraying, electroplating, electroforming, co-deposition by electroplating, electroless plating, dip coating, painting etc. Oxygen storage material 3 is then deposited on top of the anodic active catalyst 2 by various methods for example spraying, screen printing, hot pressing, sintering, thermal spraying, electroplating, electroforming, co-deposition by electroplating, electroless plating, dip coating, painting etc. The complete anode structure is then placed on one side of a porous separator 1.
[0072]An anode monopolar plate 5 is compressed to the back of the anodic perforated current collector 4 for supplying electricity and taking oxygen gas out of the c...
example 3
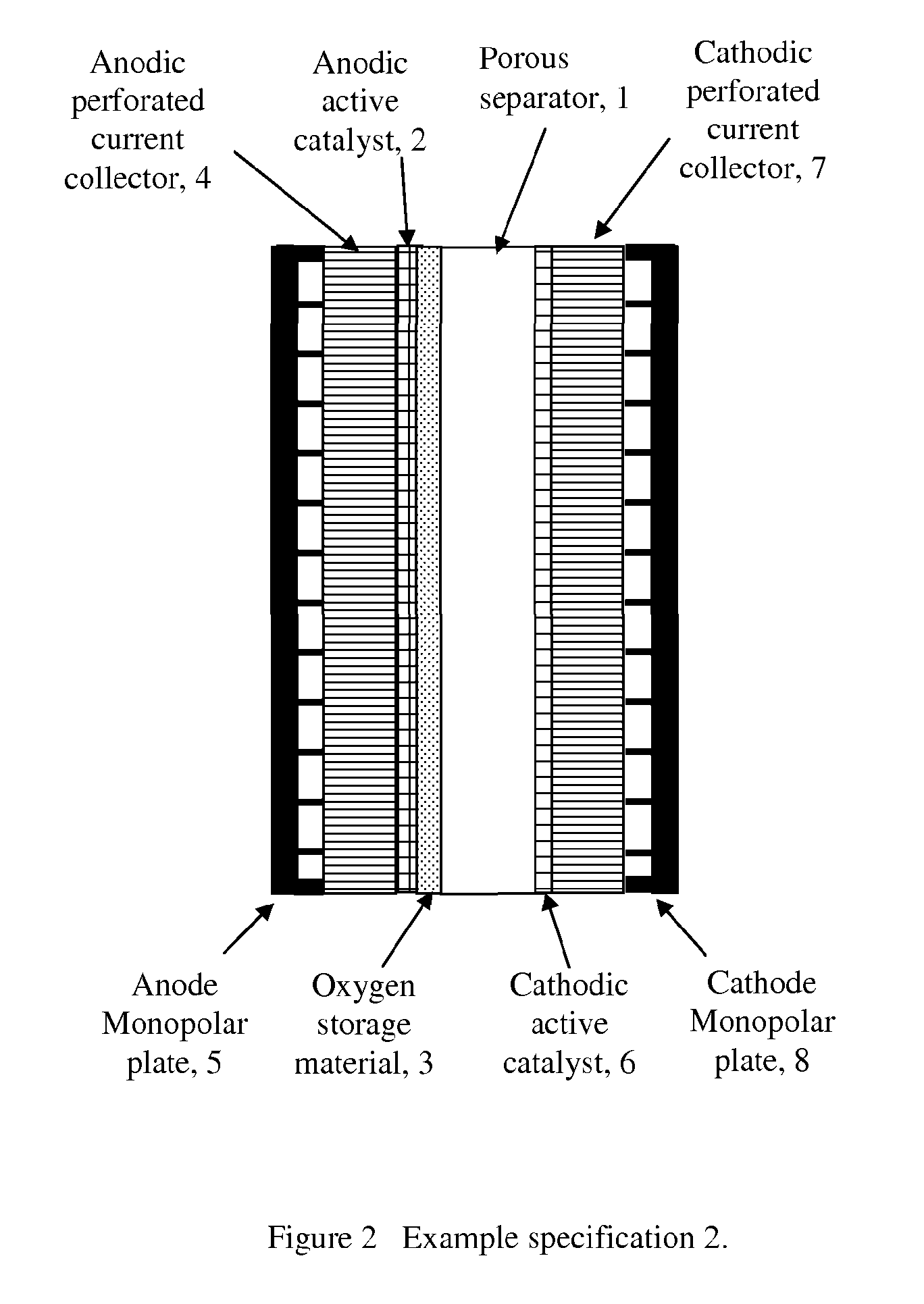
[0074]FIG. 3 shows the third example of one of the various electrode configurations described in accordance with an embodiment of the invention.
[0075]As shown in FIG. 3, an anodic active catalyst and oxygen storage material mixture 2 is deposited onto anodic perforated current collector 3 by various methods, for example spraying, screen printing, hot pressing, sintering, thermal spraying, electroplating, electroforming, co-deposition by electroplating, electroless plating, dip coating, painting etc. An anode monopolar plate 4 is compressed to the back of anodic perforated current collector 4 for supplying electricity and taking oxygen gas out of the cell.
[0076]A cathodic active catalyst 5 is deposited onto a cathodic perforated current collector 6 by various methods for example spraying, screen printing, hot pressing, sintering, thermal spraying, electroplating, electroforming, co-deposition by electroplating, electroless plating, dip coating, painting etc. A cathode monopolar plate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| open circuit voltage | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com