Method of delivering a medical device across a valve
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0069]While the disclosed subject matter may be embodied in many different forms, reference will now be made in detail to specific embodiments of the disclosed subject, examples of which are illustrated in the accompanying drawings. This description is an exemplification of the principles of the disclosed subject matter and is not intended to limit the subject matter to the particular embodiments illustrated.
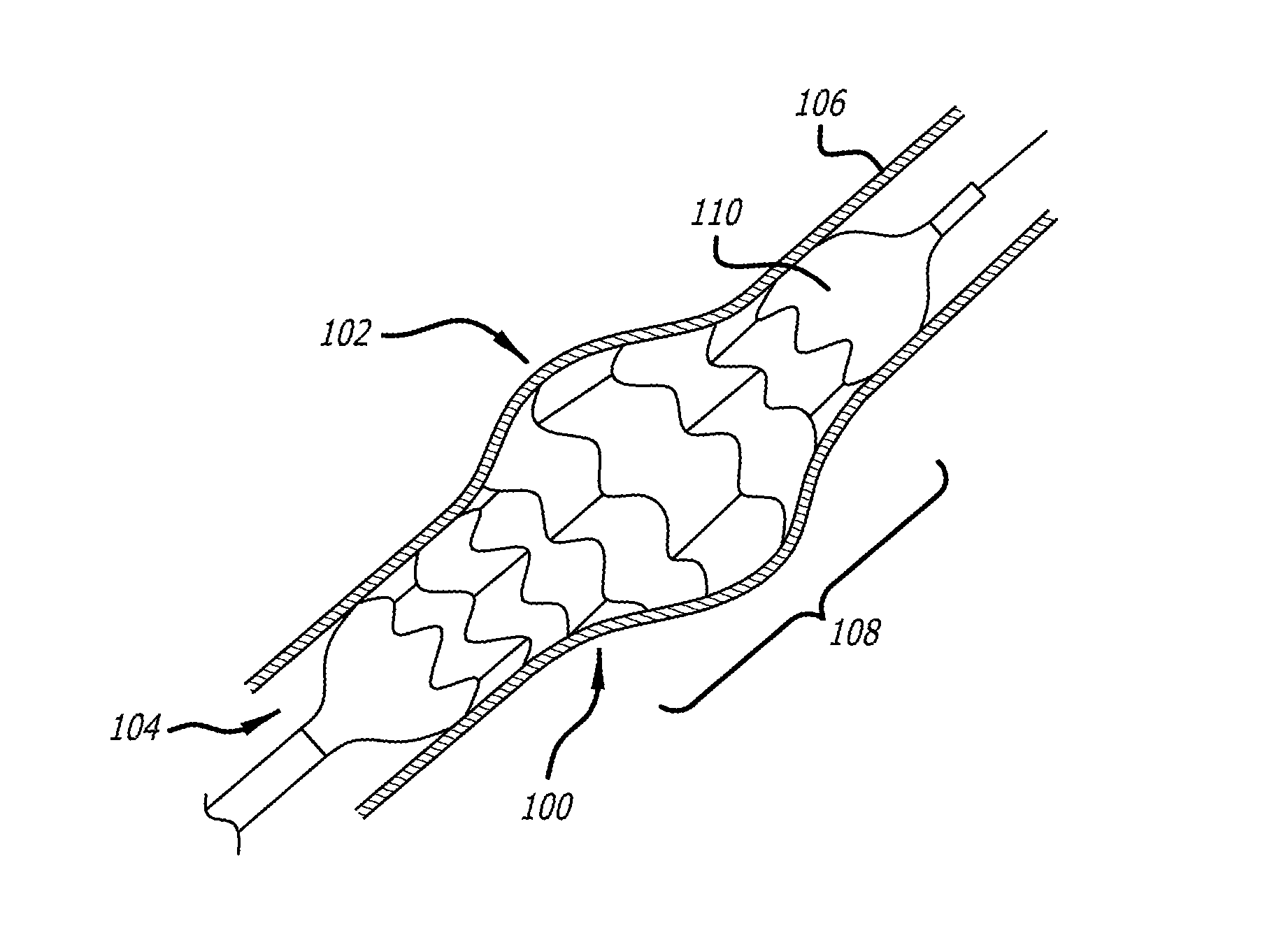
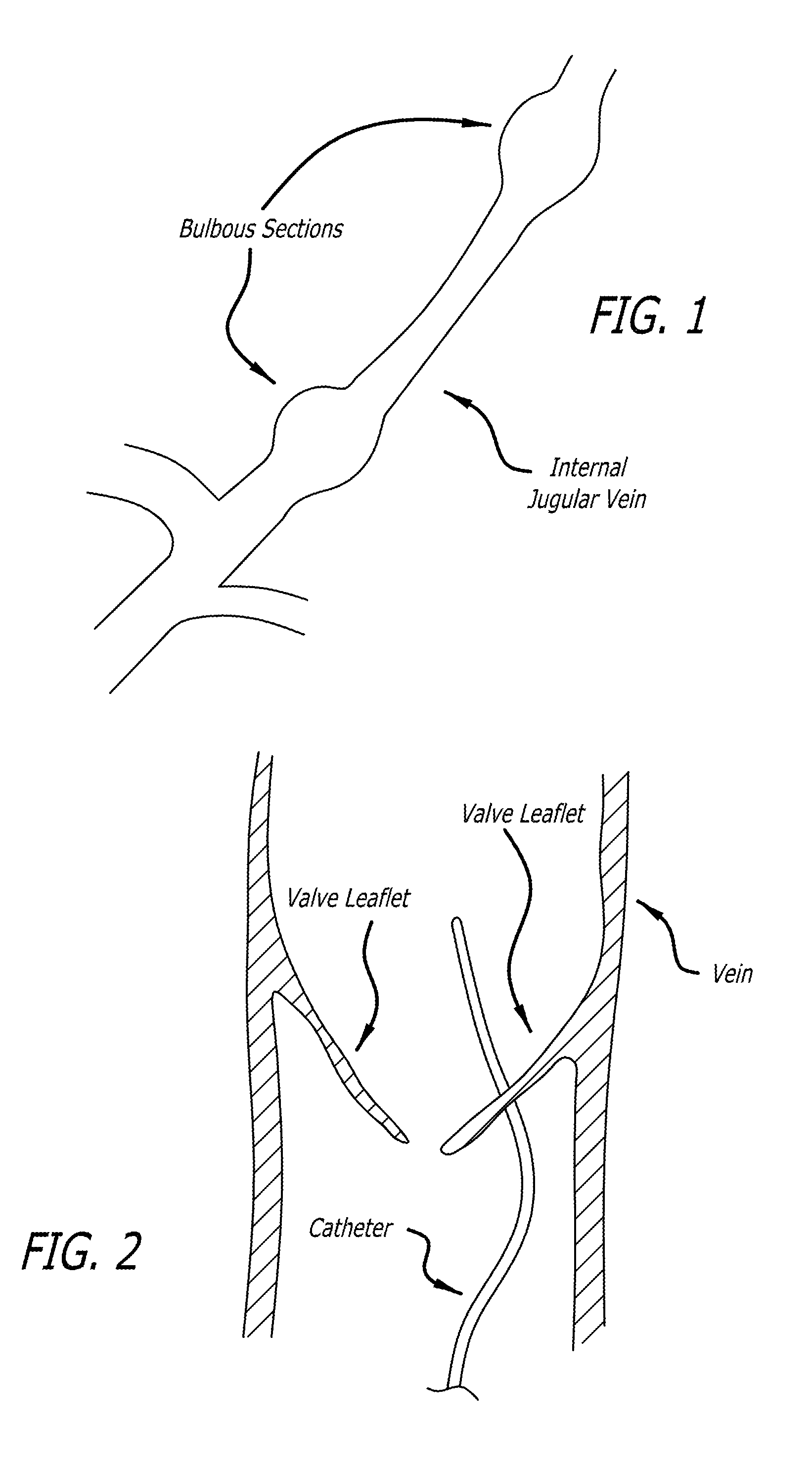
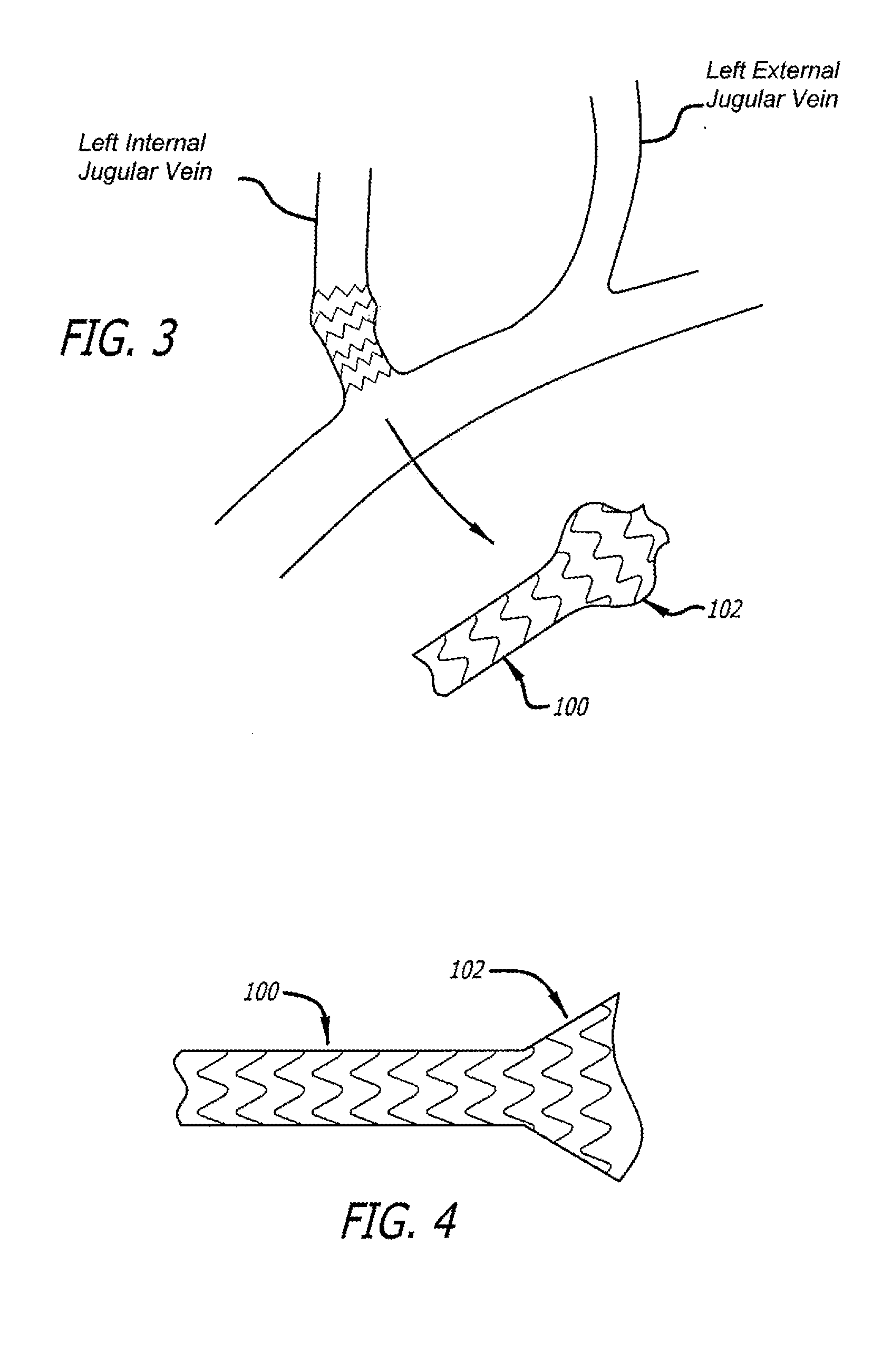
[0070]In accordance with one aspect of the disclosed matter, an intraluminal scaffold is provided which is suitable to be implanted in a body lumen, such as a blood vessel or the like, e.g., a vein, of a patient. In general, the construction of the intraluminal scaffold can be selected such that the scaffold, or a portion thereof, can either support or conform to a body lumen. By “conforming,” when the term relates to a scaffold of a portion thereof, it is intended that the overall geometry and stiffness of the scaffold, or relevant portion thereof, are such that the scaffold (o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com