Method for purifying albumin
a technology of purification method and albumin, which is applied in the field of purification method, can solve the problems of time-consuming and/or expensive, and the purification process is required
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Comparison of rHSA 2 Step AlbuPure™ Purification and 3 Step Conventional Purification
[0069]The conventional 3 step purification of cation ion exchange, anion exchange and dye affinity chromatography was performed as described in WO 2000 / 044772.
[0070]The 2 step AlbuPure™ purification was performed by conditioned the rHSA fermentation supernatant for chromatography using acetic acid and water to achieve a pH of approximately 5.3 and a conductivity of approximately 3 mS·cm−1. A column packed with AlbuPure™ matrix was equilibrated with 50 mM sodium acetate pH 5.3. A volume of conditioned sample equivalent to 20 mg rHSA·mL−1 matrix was loaded. The matrix was washed sequentially with 50 mM sodium acetate pH 5.3, 50 mM sodium phosphate pH 6.0, 50 mM sodium phosphate pH 7.0 and 50 mM ammonium acetate pH 8.0 before being eluted with 50 mM ammonium acetate, 10 mM sodium octanoate pH 7.0 and finally regenerated with 0.5M sodium hydroxide. The AlbuPure™ eluate was conditioned for anion ion exch...
example 2
rHSA Fusions
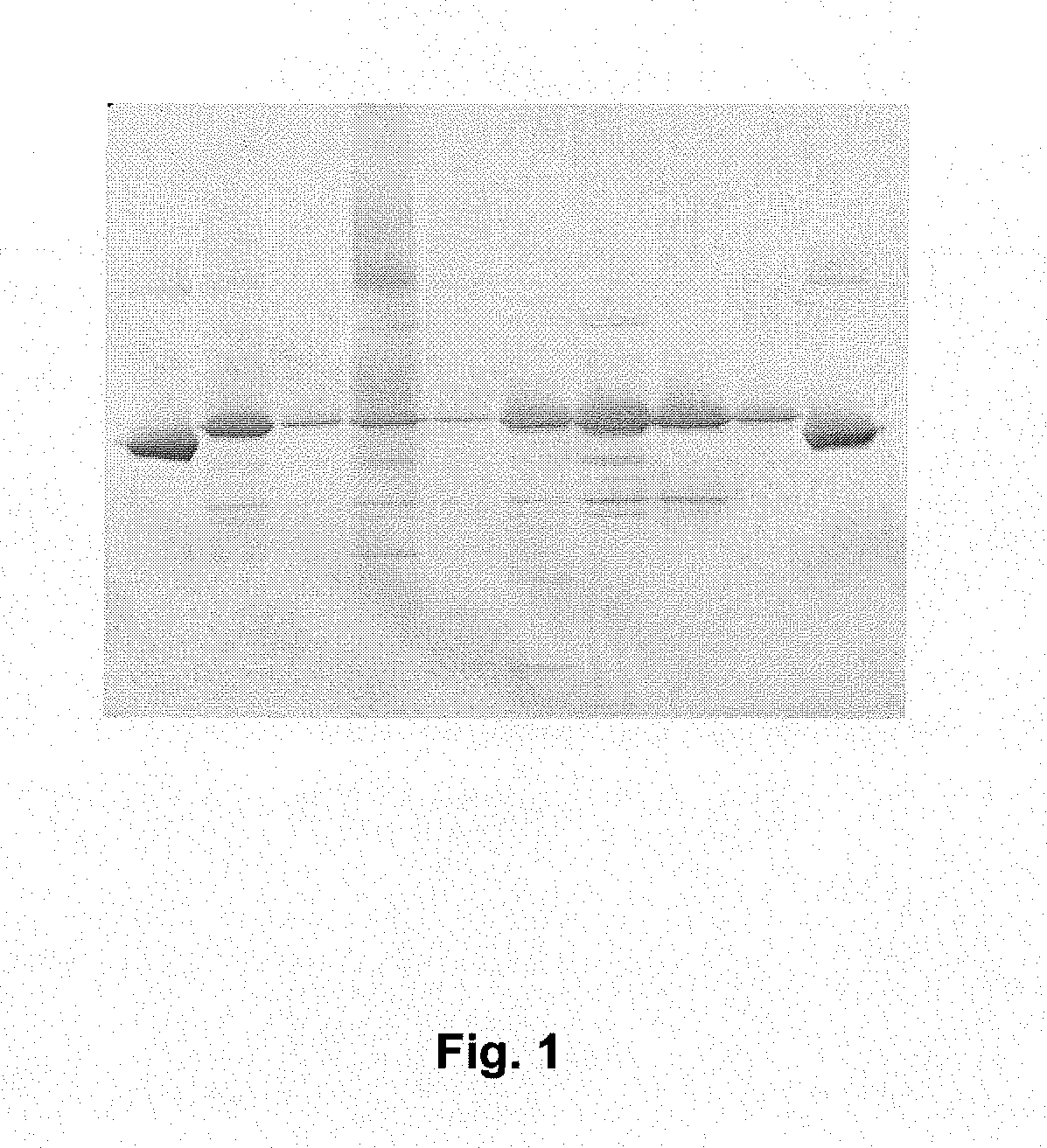
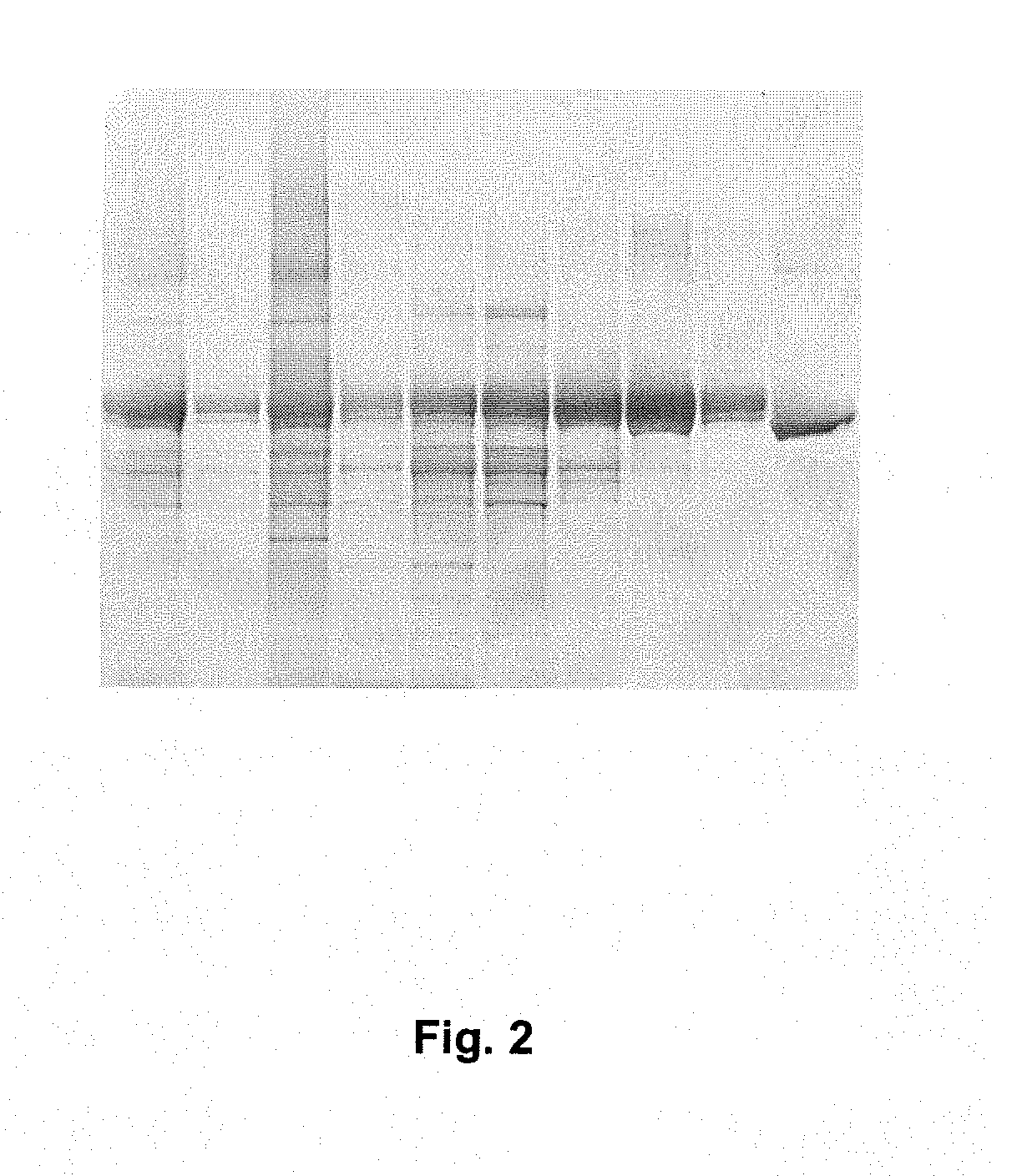
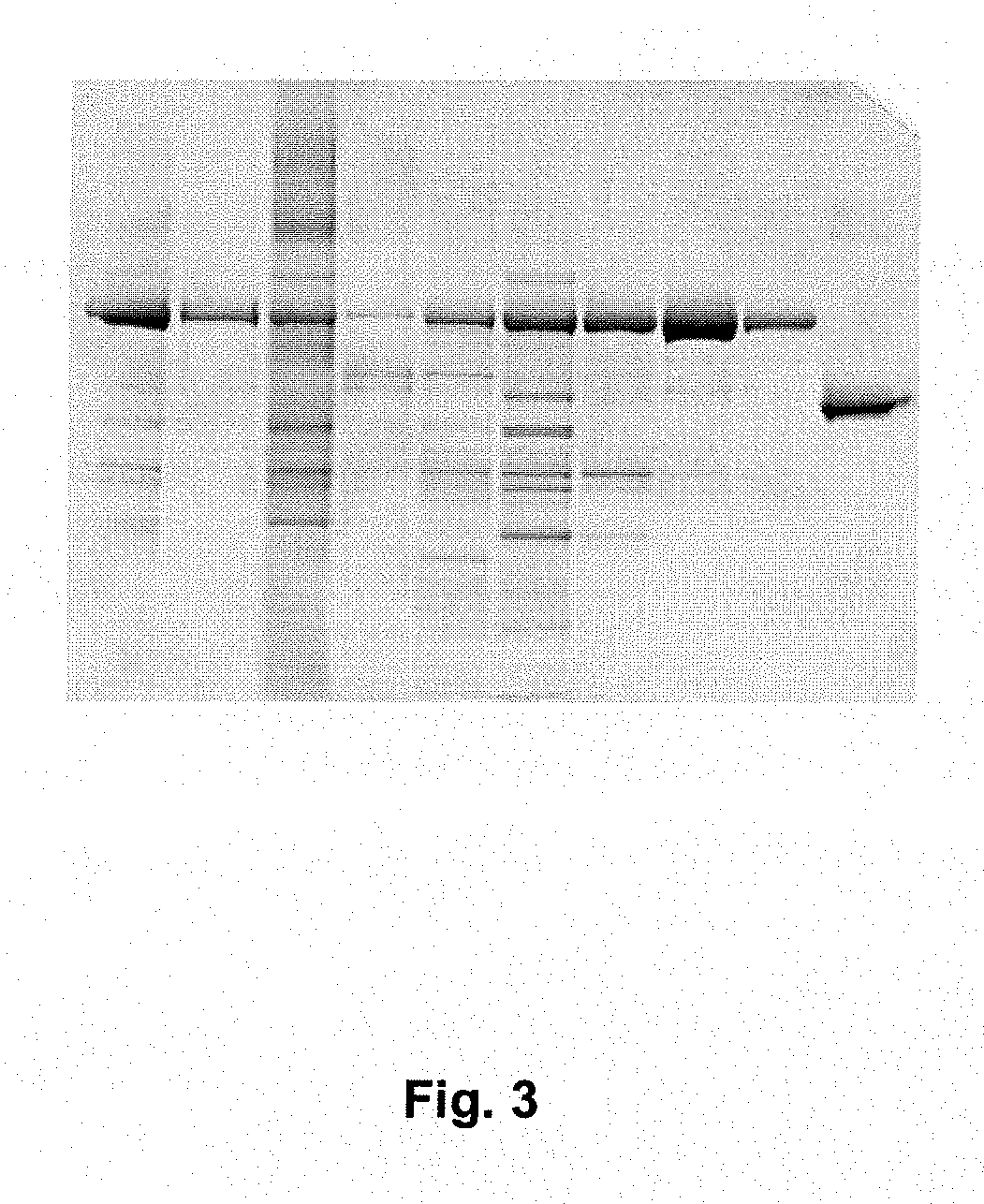
[0072]Yeast derived culture supernatant containing c-terminal rHSA fusions of prosaptide, T20 (PCT / 1B03 / 00434) and IL1RA were conditioned for chromatography using acetic acid and water to achieve a pH of approximately 5.3 and a conductivity of between 2.6 and 3.3 mS·cm−1. A 1.6 cm×11.0 cm (22.1 mL) column packed with AlbuPure™ matrix was equilibrated with 50 mM sodium acetate pH 5.3. A volume of conditioned sample equivalent to 20 mg fusion protein·mL−1 matrix was loaded. The matrix was washed sequentially with 50 mM sodium acetate pH 5.3, 50 mM sodium phosphate pH 6.0, 50 mM sodium phosphate pH 7.0 and 50 mM ammonium acetate pH 8.0 before being eluted with 50 mM ammonium acetate, 10 mM sodium octanoate pH 7.0 and finally regenerated with 0.5M sodium hydroxide. SDS_PAGE of Prosaptide albumin fusion purification is shown in FIG. 1. SDS-PAGE of T20 albumin fusion purification is shown in FIG. 2. SDS-PAGE of IL1RA Albumin fusion purification is shown in FIG. 3.
example 3
Anti FITC (scFv(vHvL)-rHSA-FLAG) Antibody
[0073]Yeast derived culture supernatant containing the anti FITC (scFv(vHvL)-rHSA-FLAG) (disclosed in EP application No 09 159 642) antibody fusion (N-terminal fusion) was conditioned for chromatography using water to achieve a pH of 6.2 and a conductivity of 7.9 mS·cm−1. A 4.4 cm×11.0 cm (167.3 mL) column packed with AlbuPure™ matrix was equilibrated with 50 mM sodium phosphate pH 6.0. A volume of conditioned sample equivalent to 9.5 mg protein·mL−1 matrix was loaded. The matrix was washed sequentially with 50 mM sodium phosphate pH 6.0, 50 mM sodium phosphate pH 7.0 and 50 mM ammonium acetate pH 8.0. Bound protein was eluted first with 50 mM ammonium acetate, 10 mM sodium octanoate pH 7.0 and then with 50 mM ammonium acetate, 30 mM sodium octanoate, 200 mM sodium chloride pH7.0. The matrix was regenerated with 0.5M sodium hydroxide. A total of 84% of the anti FITC (scFv(vHvL)-rHSA-FLAG) was recovered in the 2 eluates combined, as estimated ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com