Agent for the treatment of alopecia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
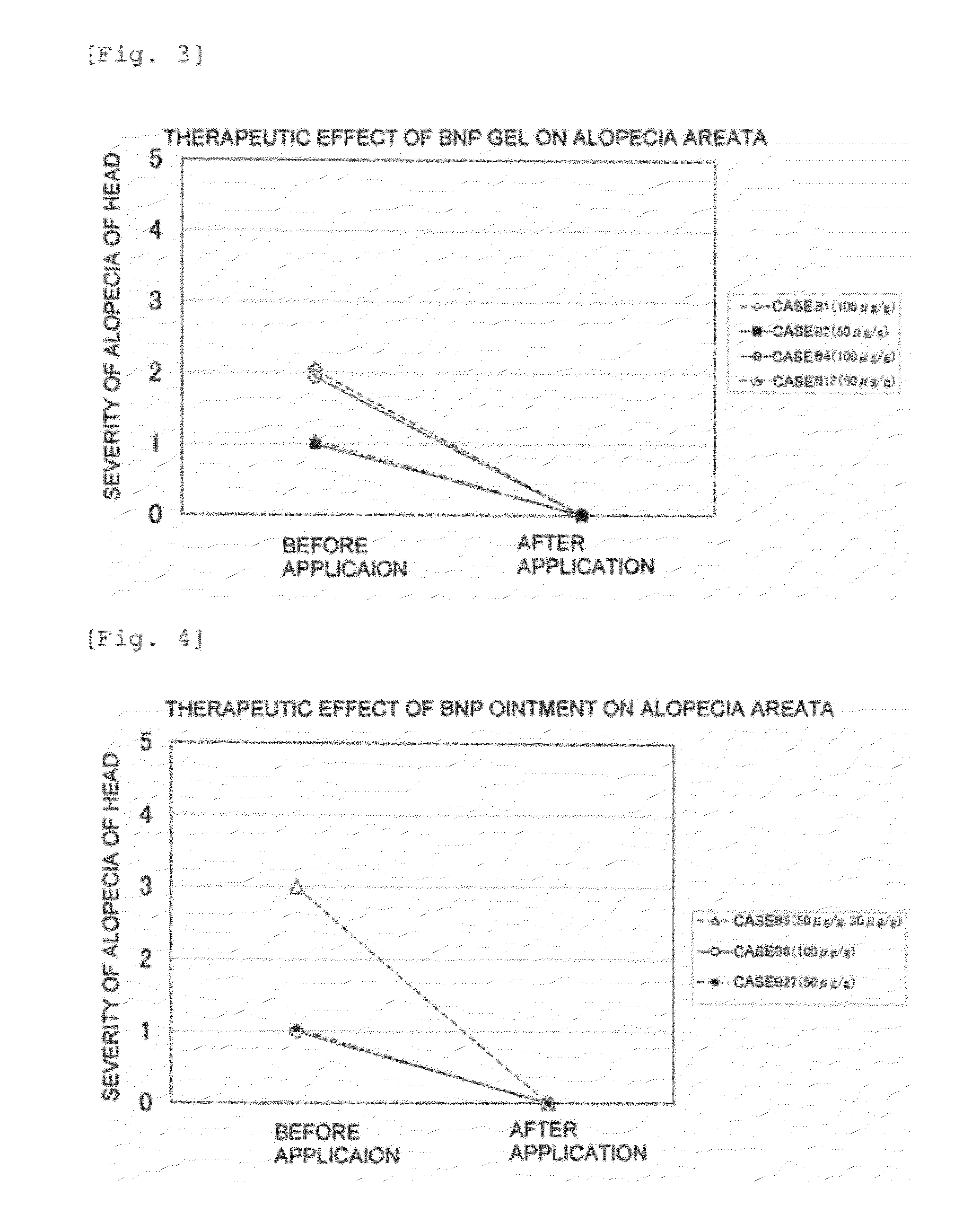
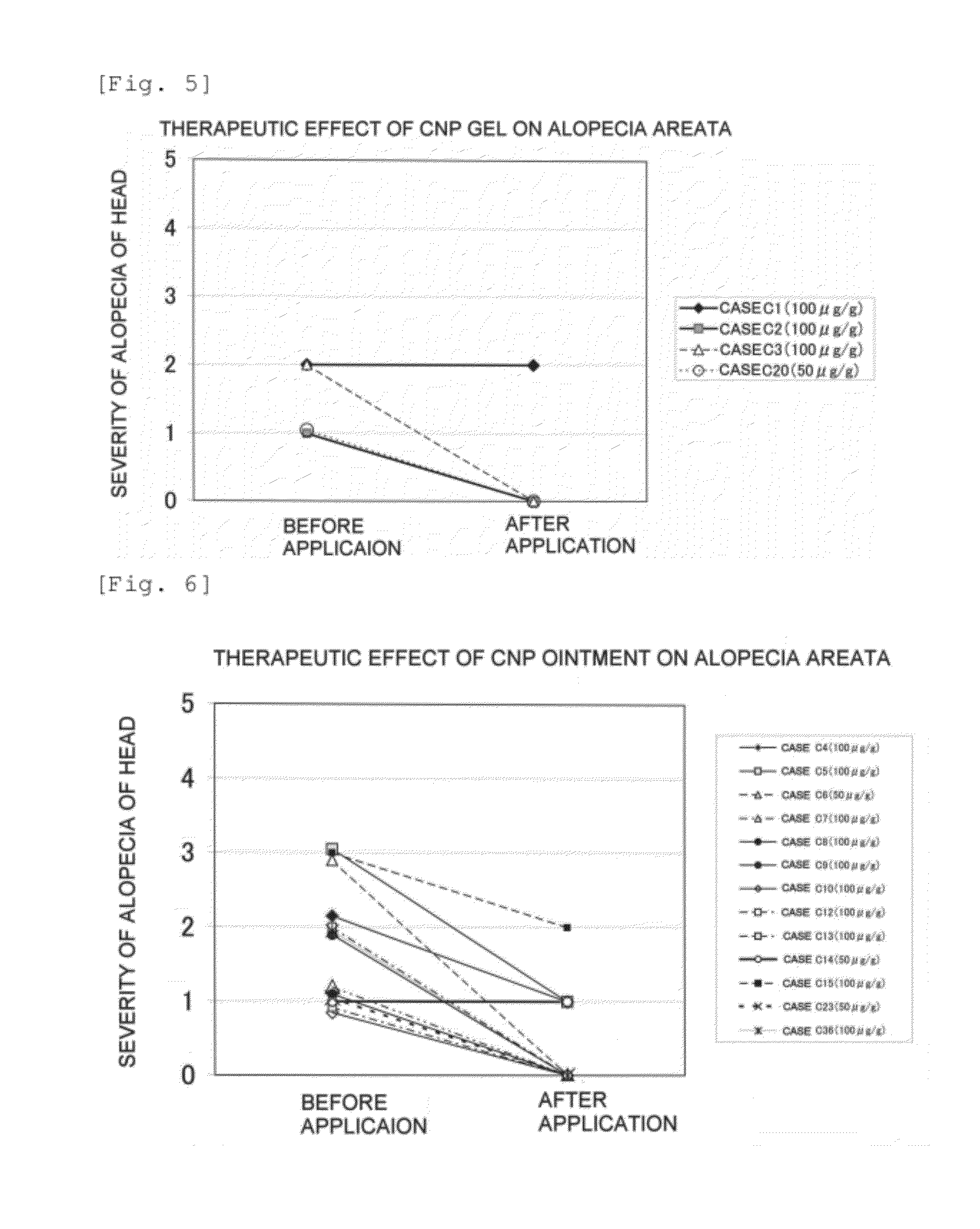
[0369]Diagnosis and evaluation of test subjects.
[0370]First, prior to administration of a natriuretic peptide preparation, diagnosis and evaluation of test subjects were carried out. Methods for the diagnosis and evaluation of test subjects were as follows.
1. Diagnosis of test subject;
[0371]The test subjects were patients with alopecia areata, androgenetic alopecia, postpartum alopecia, female pattern alopecia, alopecia pityroides, and senile alopecia. Diagnosis and treatment of these test subjects were carried out by the present inventors as physicians.
2. Evaluation of symptoms;
[0372]Clinical classification of alopecia was carried out in accordance with the Classification of the Japanese Dermatological Association, and evaluation of the severity of alopecia areata was carried out in accordance with the above-mentioned ‘USA Alopecia Areata Evaluation Guidelines’, the area of hair loss being classified using 6 grades of S0 to S5 and the hair loss site being classified using 3 grades ...
example 2
1. Production of Gel
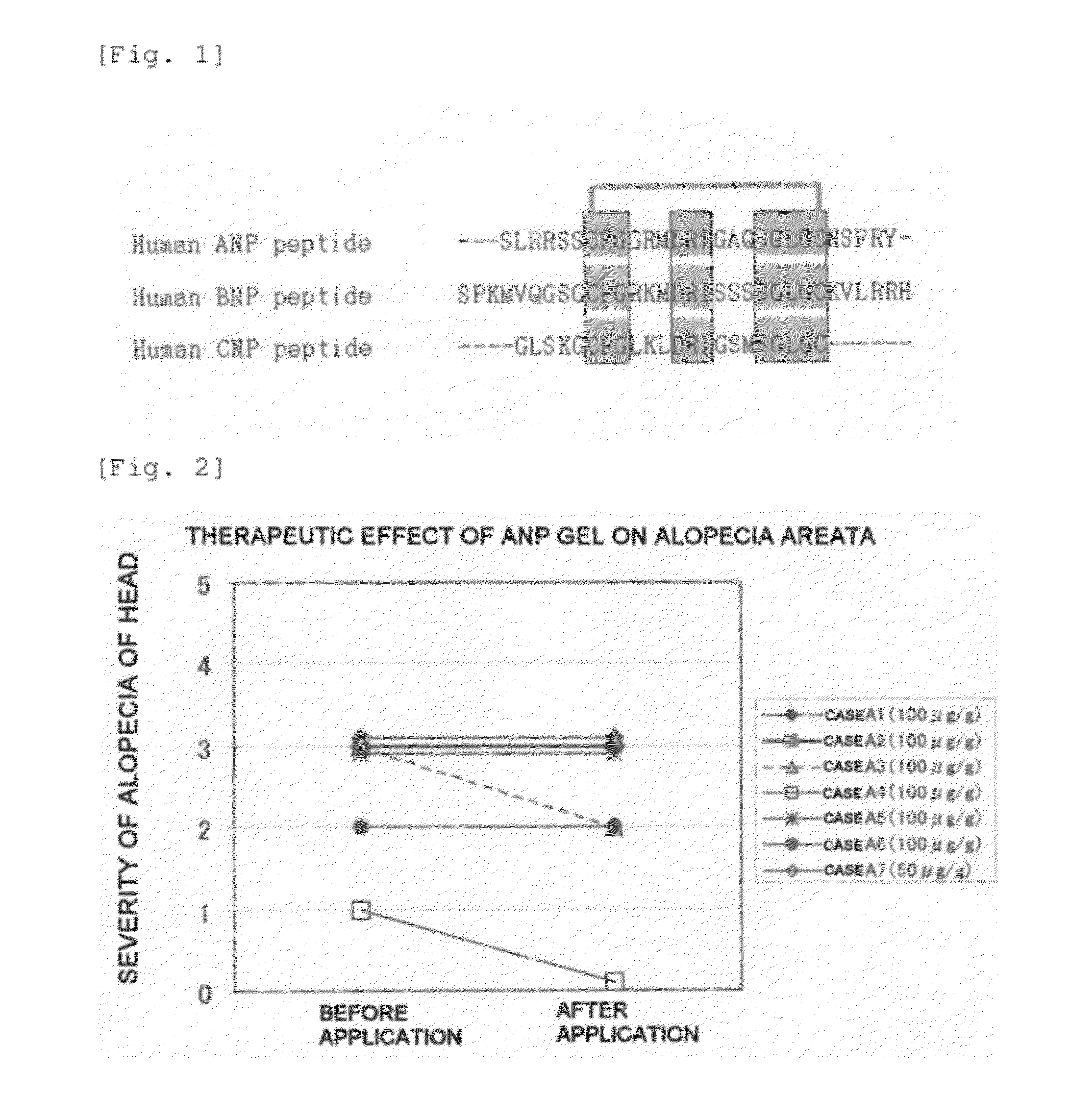
[0374]Preparation of a gel containing an NP as an active ingredient was carried out by weighing, as a main agent, 3 mg of any one of human ANP (1-28) (Peptide Institute, Inc.), human BNP-32 (Peptide Institute, Inc.), and human CNP-22 (Peptide Institute, Inc.), dissolving this in 3 mL of purified water to give an NP solution having a concentration of 1000 μg / mL, and mixing 1 mL of this solution with 9 g of Lubrajel NP (ISP Japan Ltd.) by uniform stirring, thus giving an ANP gel, a BNP gel, and a CNP gel having a concentration of 100 μg / g.
[0375]Similarly, 500 μL of the NP solution having a concentration of 1000 μg / mL obtained above was diluted with 500 μL of physiological saline to thus adjust the concentration to 500 μg / mL, and 1 mL of this solution was mixed with 9 g of Lubrajel NP (ISP Japan Ltd.) by uniformly stirring, thus giving an ANP gel, a BNP gel, and a CNP gel having a concentration of 50 μg / g.
2. Production of Ointment (Vaseline Preparation)
[0376]Prepara...
example 3
1. Diagnosis and Treatment of Test Subjects
[0385]Prior to administration of the ANP gel, BNP gel, CNP gel, BNP ointment, and CNP ointment of the present invention, as is routine for dermatological diagnosis and treatment, the test subjects were interviewed regarding age, gender, history of disease, and family history of disease; when an allergic predisposition was suspected, a scratch test against the main allergens and an evaluation thereof were carried out. In dermatological diagnosis and treatment, since there are a relatively large number of patients who have strong immunoreactivity toward a specific allergen, or a disease such as atopic dermatitis that is suspected to be related to having an allergic predisposition, in order to assist diagnosis, an interview with respect to family history and previous history of allergic disease in addition to gender and age, and a scratch test toward the main allergens, are widely carried out as simple supplemental test methods.
[0386]With rega...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Dimensionless property | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com