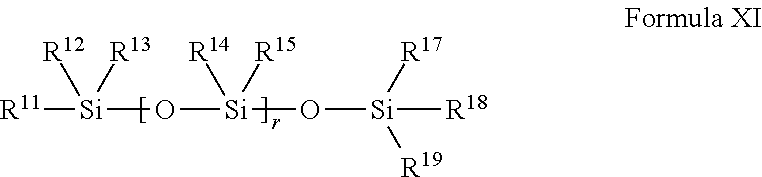
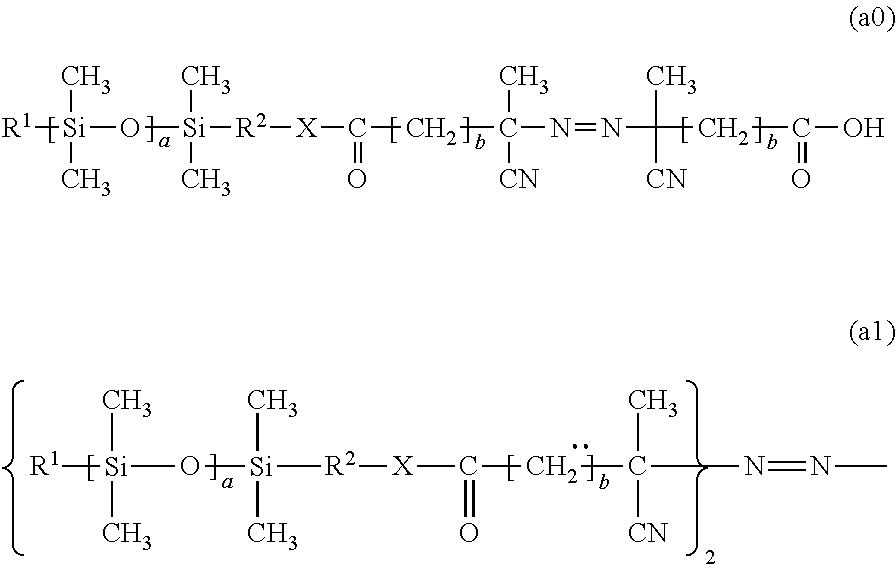Macroinitiator containing hydrophobic segment
a technology of hydrophobic segments and micro-initiators, which is applied in the field of micro-initiators, can solve the problems that large polysiloxane segments are difficult to solubilize in aqueous solutions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
working example 1
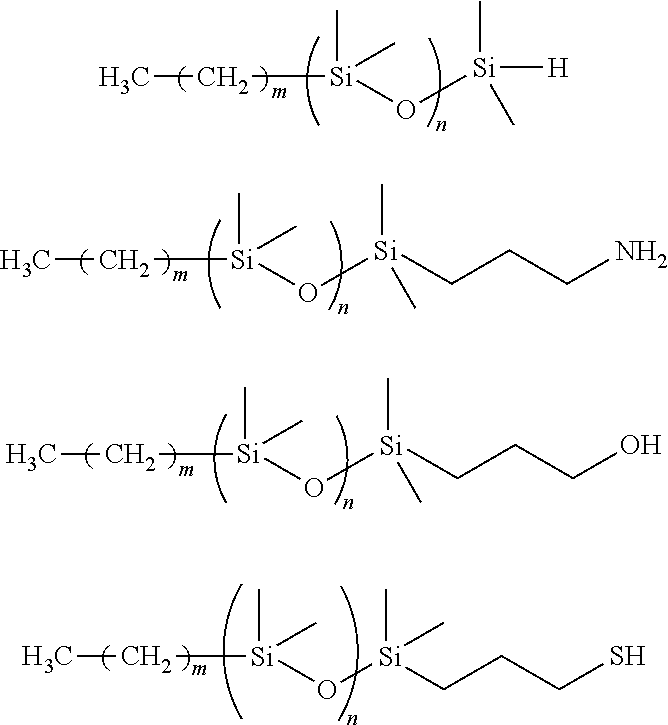
[0082]1.68 g (6 mmol) of 4,4′-azobis(4-cyanovalearic acid) and 1.83 g (15 mmol) of 4-dimethyl amino pyridine, 3.0 g (15 mmol) of N,N-dicyclohexyl carbodiimide, and 40 mL of acetone were added to a 200 mL three mouth flask equipped with a calcium chloride tube under nitrogen gas flow. 8.58 g (9 mmol) of polydimethylsiloxane having a hydroxyl group at one end and expressed by the following formula (a2)
(manufactured by Chisso Corporation FM-0411, Mw 1000) was added by drops to the solution and agitated for six hours at room temperature. A precipitated solid was filtered out, hexane was added to the filtrate obtained, and then the filtrate was washed two times with 0.5 N HCl, two times with saturated sodium bicarbonate aqueous solution, and one time with saturated sodium chloride aqueous solution. The organic phase was dried using sodium sulfate, filtered, and then concentrated to obtain crude product. The crude product was purified using a silica gel column (silica gel 180 g, hexane / et...
working example 2
[0083]1.40 g (5 mmol) of 4,4′-azobis(4-cyanovalearic acid), 9.1 g (9.1 mmol) of polydimethylsiloxane having an amino group at one end and expressed by the following formula (a3) (manufactured by Chisso Corporation, FM0311, Mw 1000), 0.67 g (5.5 mmol) of 4-dimethyl aminopyridine, and 50 mL of acetone were added to a 200 mL three mouth flask equipped with a calcium chloride tube under nitrogen gas flow.
1.70 mL (11 mmol) of N,N-diisopropyl carbodiimide was added by drops to this blended solution. After agitating for 6 hours at ambient temperature, a precipitated solid was filtered out, hexane was added to the filtrate obtained, and then the filtrate was washed two times with 0.5 N HCl, two times with saturated sodium bicarbonate aqueous solution, and one time with saturated sodium chloride aqueous solution. The organic phase was dried using sodium sulfate, filtered, concentrated, and then the crude product was purified using a silica gel column (silica gel 180 g, hexane / ethyl acetate=1...
working example 3
[0086]N-vinyl pyrrolidone (NVP, 29.56 g, 0.266 mol), the silicone macro initiator expressed by the following formula (a4) obtained by working example 1 (Mw of silicone portion is 1000, 0.19 g, 0.0866 mmol), and t-amyl alcohol (TAA, 69.42 g) were added to a 200 mL three mouth flask, and then a three way cock, thermometer, and mechanical stirrer were attached.
The inside of the three mouth flask was evacuated using a vacuum pump and then substituted with argon, three times, and then the temperature was increased to 70° C. After confirming that the temperature had stabilized and heat generation was not occurring, the temperature was increased to 75° C. and the sample was agitated for 6 hours.
[0087]After polymerization was complete, the temperature was cooled to room temperature, and then the sample was poured into n-hexane / ethanol=500 mL / 40 mL and allowed to sit. The supernatant fluid was removed by decanting, and then the washing was performed 2 times using n-hexane / ethanol=500 mL / 20 m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight % | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com