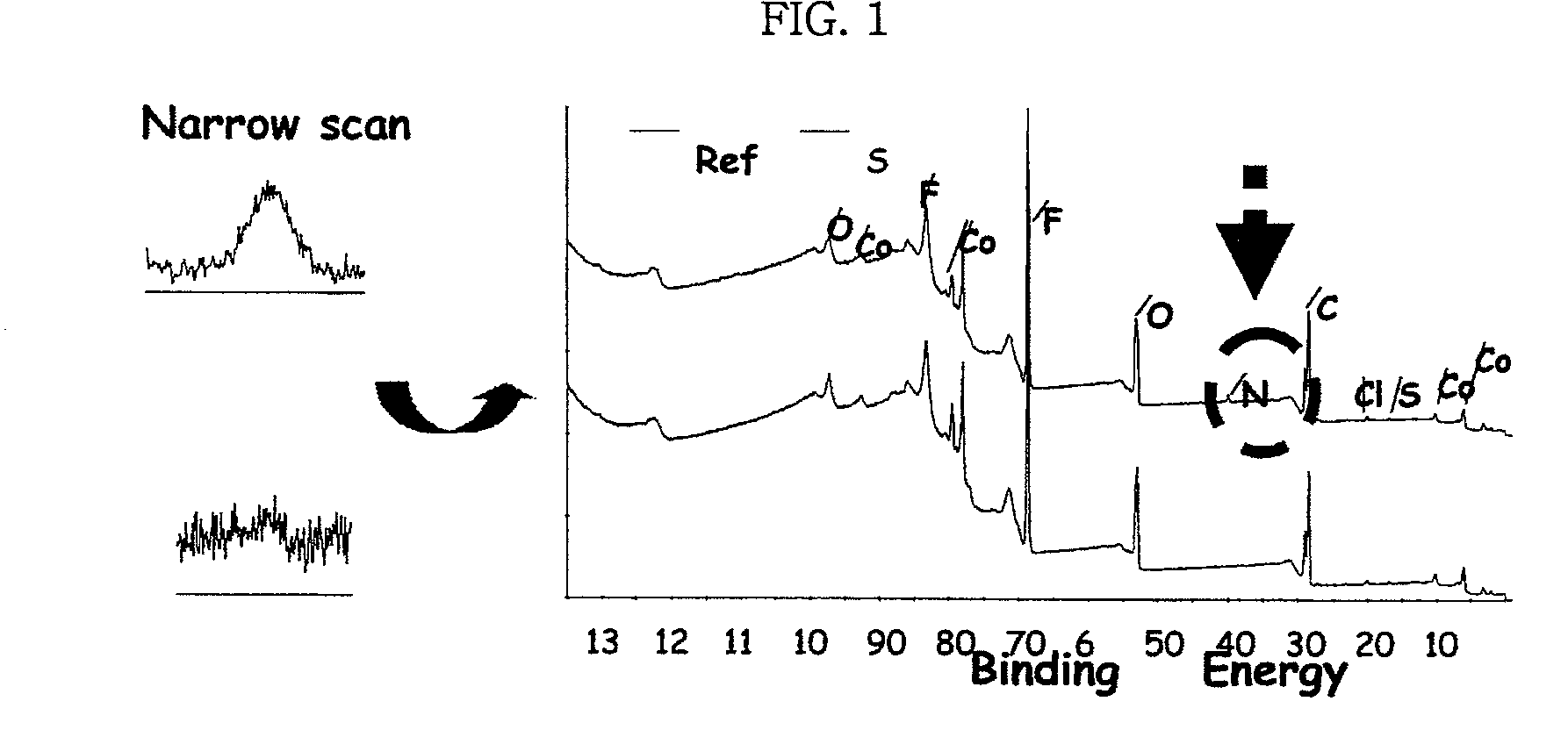
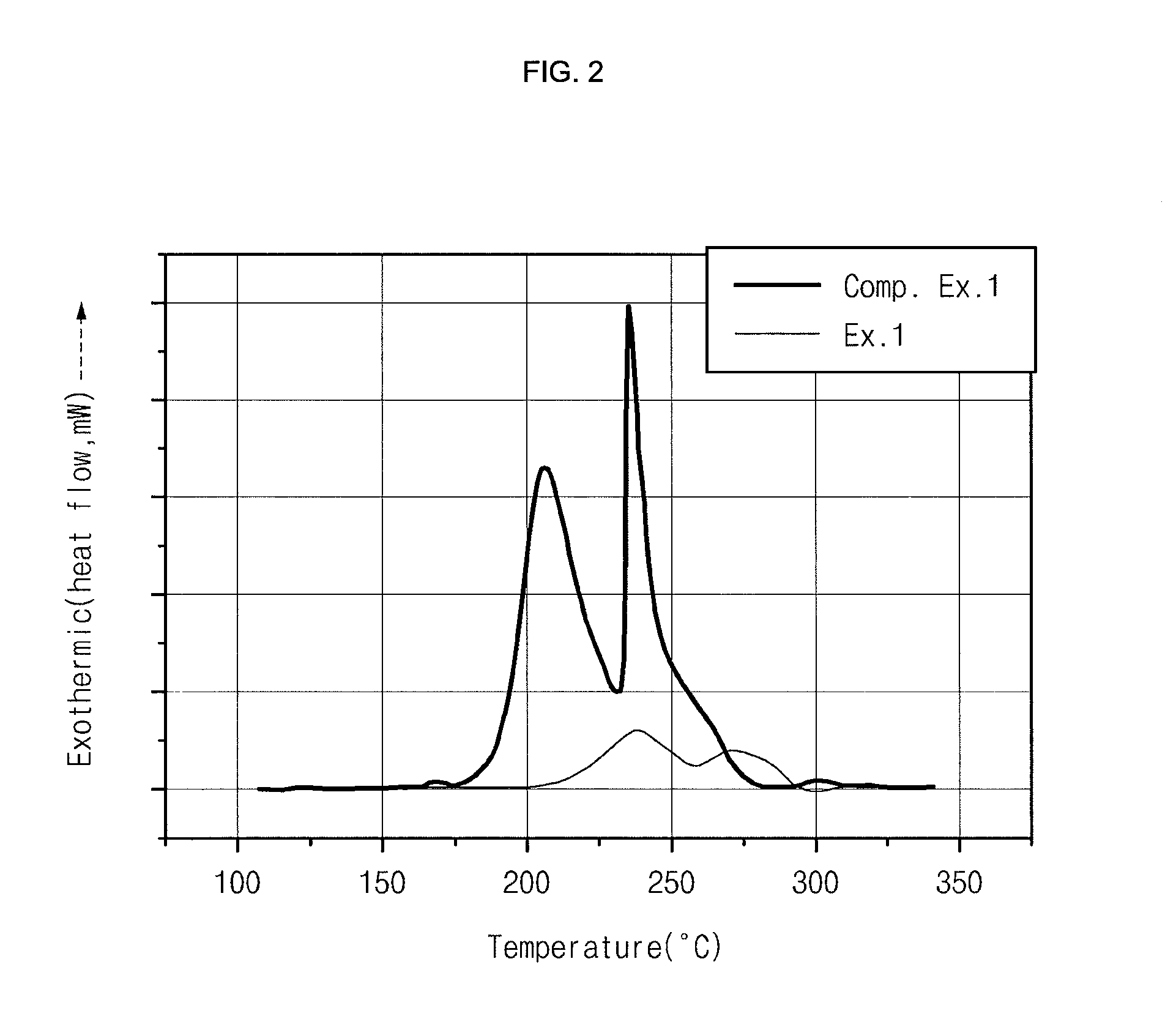
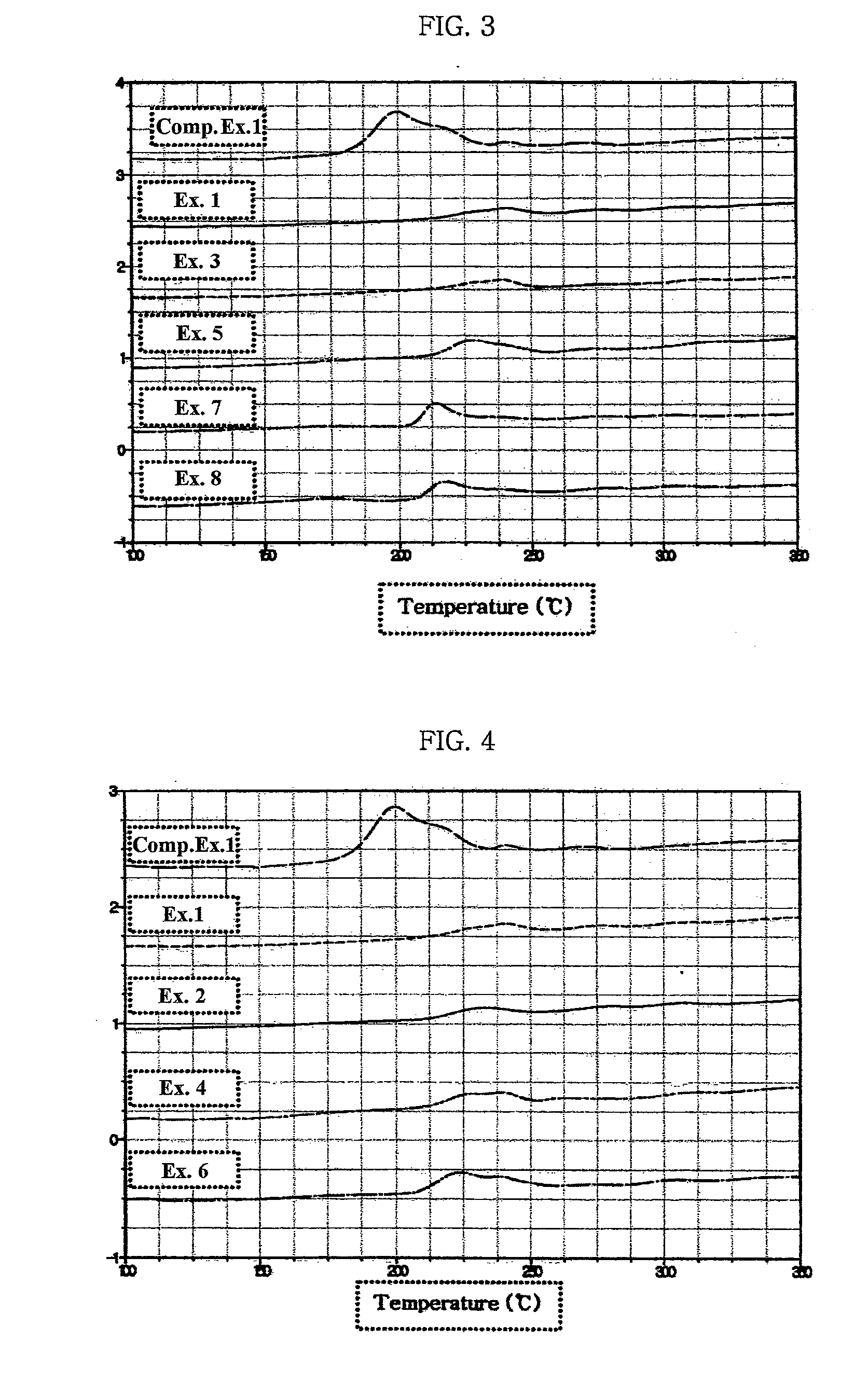
Electrode for lithium secondary battery
a lithium secondary battery and electrode technology, applied in the field of electrodes, can solve the problems of increasing the viscosity of the electrolyte, affecting the performance of the battery at a low temperature, and the diffusion of li ions cannot be smoothly made under extreme conditions, so as to increase the irreversible capacity, reduce the risk of li ions being released, and improve the effect of battery performan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0062]Succinonitrile was diluted with acetone as a solvent in the weight ratio of 3:7 to provide a solution, into which a cathode was dipped. Then, the cathode was high-temperature treated at 30° C. for 2 days to evaporate the solvent, thereby providing a cathode comprising succinocitrile forming a complex with the surface of cathode active material. The cathode active material was LiCoO2. Artificial graphite was used as an anode active material. The electrolyte used in this example was 1M LiPF6 solution formed of EC:PC:DEC=3:2:5. A 383562-type lithium polymer battery was manufactured by using a conventional method and the battery was packed with an aluminum laminate packaging material to provide a battery pack. Next, the battery was aged by treating it again at a high temperature of 60° C. for 12 hours or more so that any unreacted and / or residual succinonitrile in the electrode can form a complex.
examples 2-8
[0063]Example 1 was repeated to provide lithium polymer batteries, except that glutaronitrile (R═C3H6) (Example 2), adiponitrile (R═C4H8) (Example 3), pimelonitrile (R═C5H10) (Example 4), octanedinitrile (R═C6H12) (Example 5), azelonitrile (R═C7H14) (Example 6), sebaconitrile (R═C8H16) (Example 7) and dodecanedinitrile (R═C10H20) (Example 8) were used, instead of succinonitrile (R═C2H4).
example 9
[0066]A cathode was dipped into a solution containing succinonitrile (R═C2H4) in a acetone as a solvent, and then was high-temperature treated at 30° C. for 2 days to evaporate the solvent, thereby providing a cathode comprising 3-5 wt % of succinocitrile, based on the weight of electrolyte, forming a complex with the surface of cathode active material. The cathode active material was LiCoO2. The electrolyte used in this example was 1M LiPF6 solution formed of EC:EMC=1:2. Artificial graphite was used as an anode active material. A 523450-type prismatic lithium battery was manufactured by using a conventional method. Next, the battery was aged by treating it again at a high temperature of 60° C. for 12 hours or more so that any unreacted and / or residual succinonitrile in the electrode can form a complex.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com