Use of nitrated lipids for treatment of side effects of toxic medical therapies
a technology of toxic medical therapy and nitrate lipids, which is applied in the direction of anhydride/acid/halide active ingredients, biocide, organic chemistry, etc., can solve the problems of increasing glomerular permeability, limited clinical use of cisplatin, and toxicity in normal tissues of both chemotherapy and radiotherapy, so as to reduce side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Evaluation of the Therapeutic Potential for Using Nitrated Fatty Acid OA-NO2 in Managing Chemotherapy-Related Toxicity
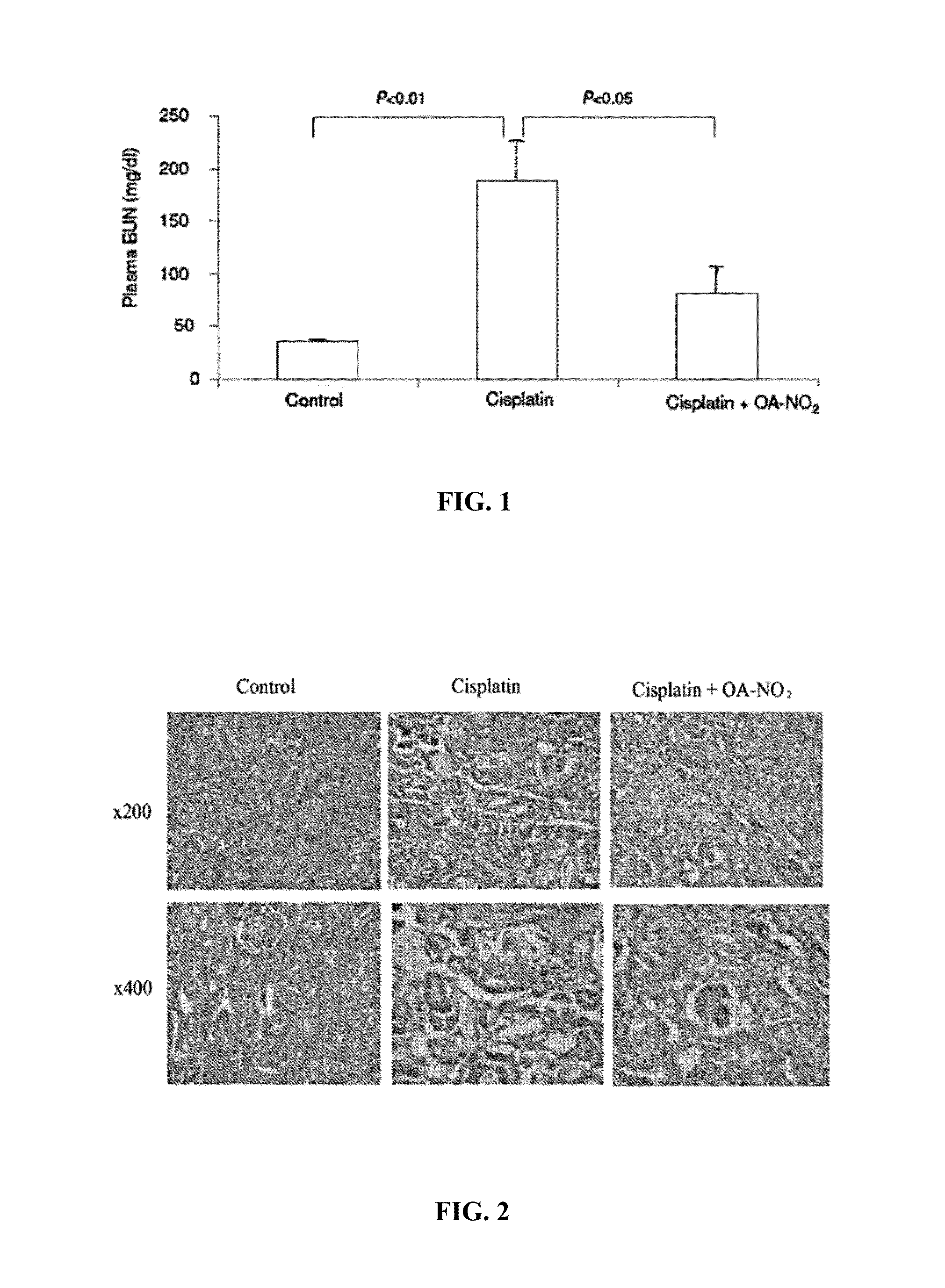
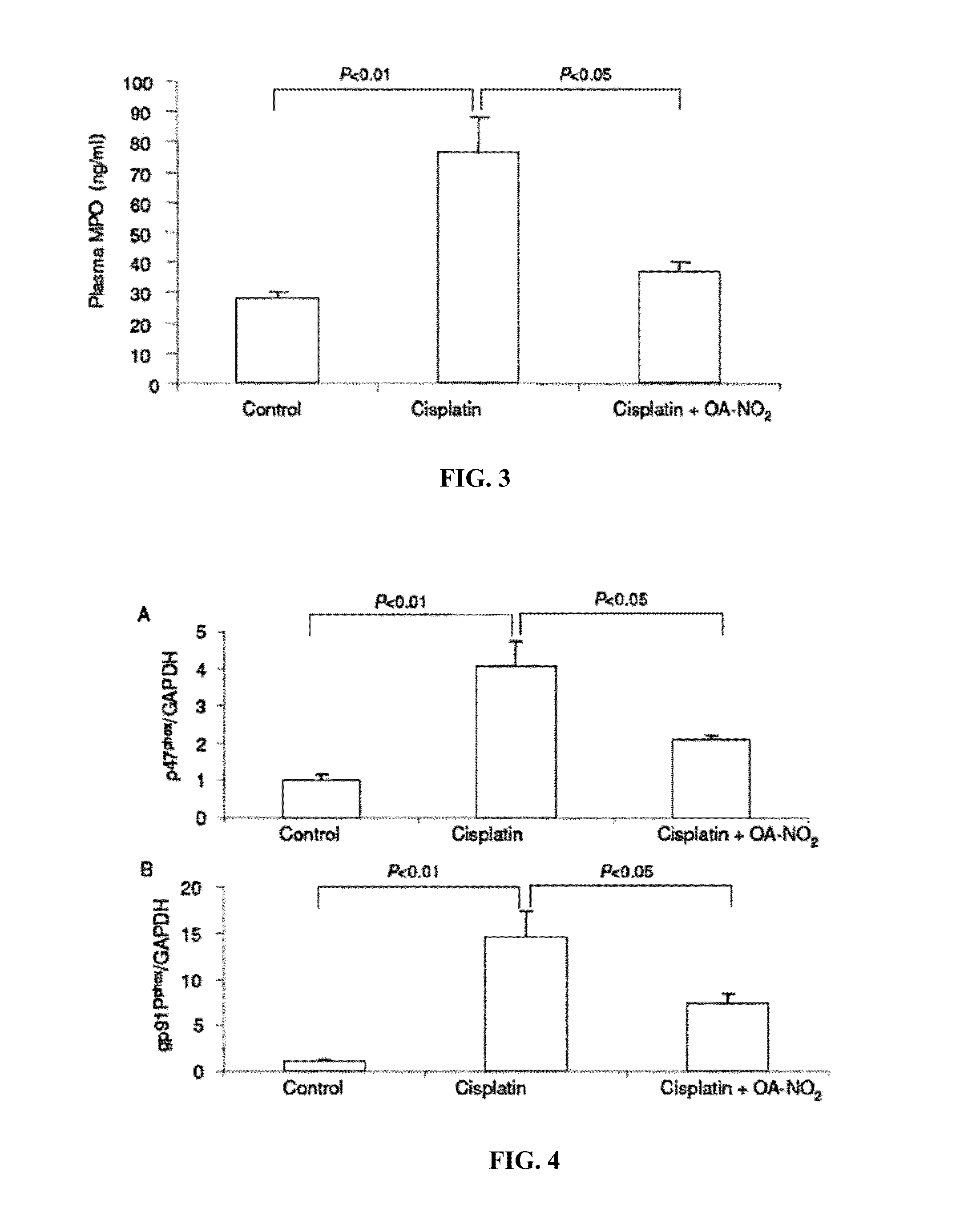
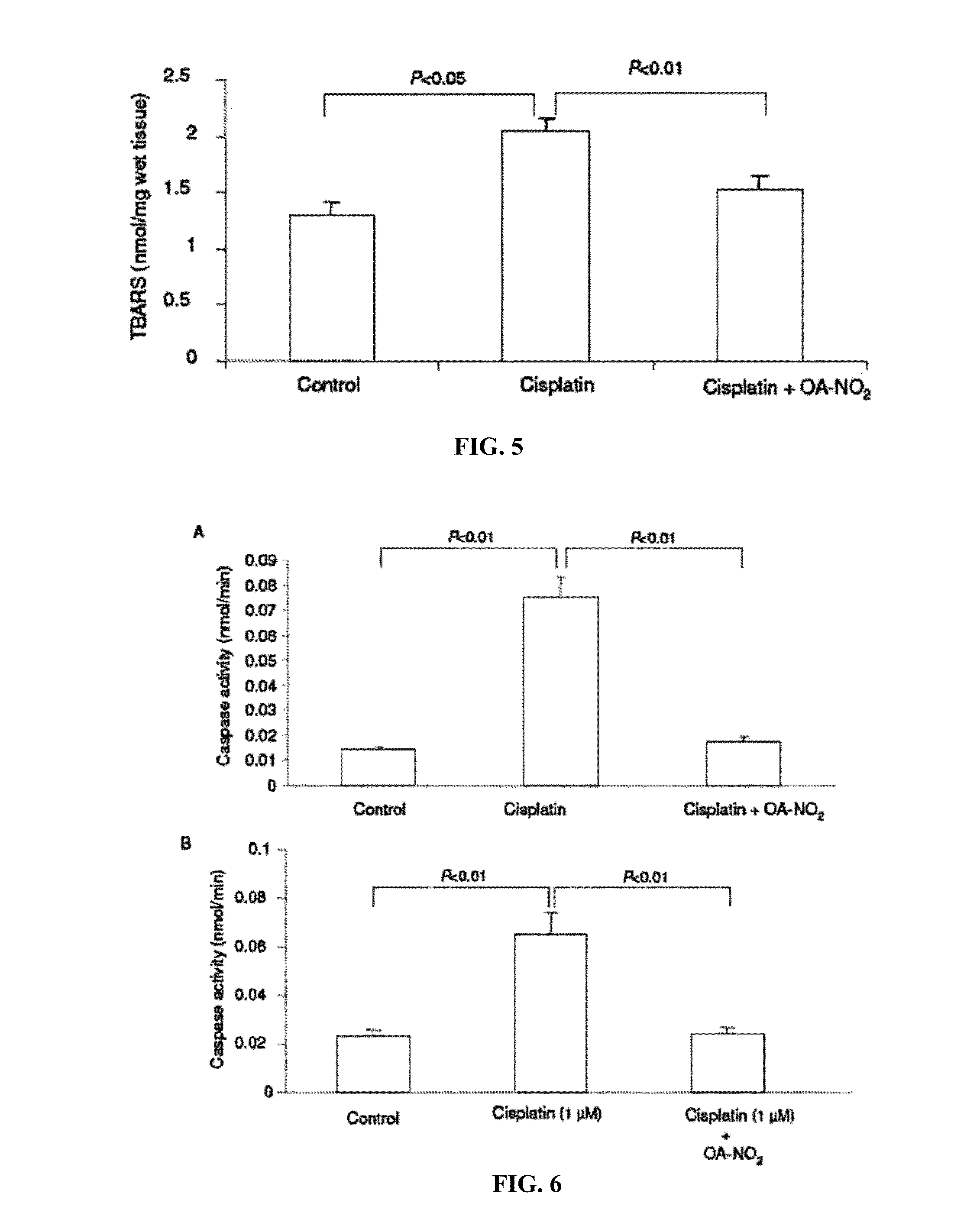
[0054]A single dose of i.p. injection of cisplatin induced renal dysfunction as indicated by the marked rise in plasma BUN (FIG. 1), accompanied by severe renal histological abnormalities characterized by distortion of the overall renal morphology, dilation of renal tubules, and appearance of protein cast (FIG. 2). In a sharp contrast, posttreatment with OA-NO2 markedly attenuated these functional and pathological changes (FIGS. 1-2). Cisplatin treatment induced increases in plasma level of MPO (marker of neutrophil infiltration) (FIG. 3), kidney expression of NADPH oxidase subunits p47phox and gp91phox (major superoxide generating enzyme) (FIG. 4), kidney thiobarbituric acid-reactive substances (TBARS, index of oxidative stress) (FIG. 5), and activity of caspase (index of apoptosis) (FIG. 6A), all of which were attenuated or completely corrected by OA-NO2. In cultur...
example 2
OA-NO2 Attenuates Albuminuria and Renal Dysfunction in Managing Chemotherapy-Related Toxicity
[0063]BALB / c mice were administered vehicle, ADR, or ADR in combination of OA-NO2; OA-NO2 was delivered via osmotic mini-pump 2 days prior to ADR injection. At day 5 after ADR injection, albuminuria was most evident in ADR group (508.89±48.52 μg / 24 h) as compared with control group (33.39±3.50 μg / 24 h), and was attenuated in ADR+OA-NO2 group (342.40±33.26 μg / 24 h). These changes were observed at day 3 and maintained at day 7. At day 7, plasma albumin was significantly reduced in the ADR group (0.28±0.08 g / dl) as compared with control group (1.01±0.15 g / dl) and was significantly restored in ADR+OA-NO2 group, the decrease of plasma albumin levels were significantly attenuated (0.58±0.13 g / dl), as shown in FIG. 8A. Ninety percent of ADR mice had severe ascites at sacrifice contrasting to only 20% of ADR+OA-NO2 mice having mild ascites, as shown in FIG. 8B.
[0064]ADR mice developed severe hyperli...
example 3
OA-NO2 Attenuates Glomerular Injury and Renal Fibrosis in Managing Chemotherapy-Related Toxicity
[0065]To correlate the reduction of albuminuria to glomerular structure, the effect of drug treatments on glomerulosclerosis was assessed by periodic acid-Schiff (PAS) staining. Being consistent with the data on albuminuria, the ADR mice showed marked glomerulosclerosis as evidenced by mesangial expansion and increased accumulation of extracellular matrix (ECM) in the mesangium, as shown in FIG. 8A. A semiquantitative glomerulosclerotic index of kidney sections confirmed the histological data. The ADR mice showed the highest score, and OA-NO2 treatment led to a marked reduction in the index (P<0.05), as shown in FIG. 8B.
[0066]Because podocyte injury is an early and predominant pathologic feature of ADR model, expression of a number of podocyte markers was examined. WT1 is a pivotal transcription factor that is essential for the maintenance of the differentiated features of adult podocytes...
PUM
| Property | Measurement | Unit |
|---|---|---|
| oxidative stress | aaaaa | aaaaa |
| permeability | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com