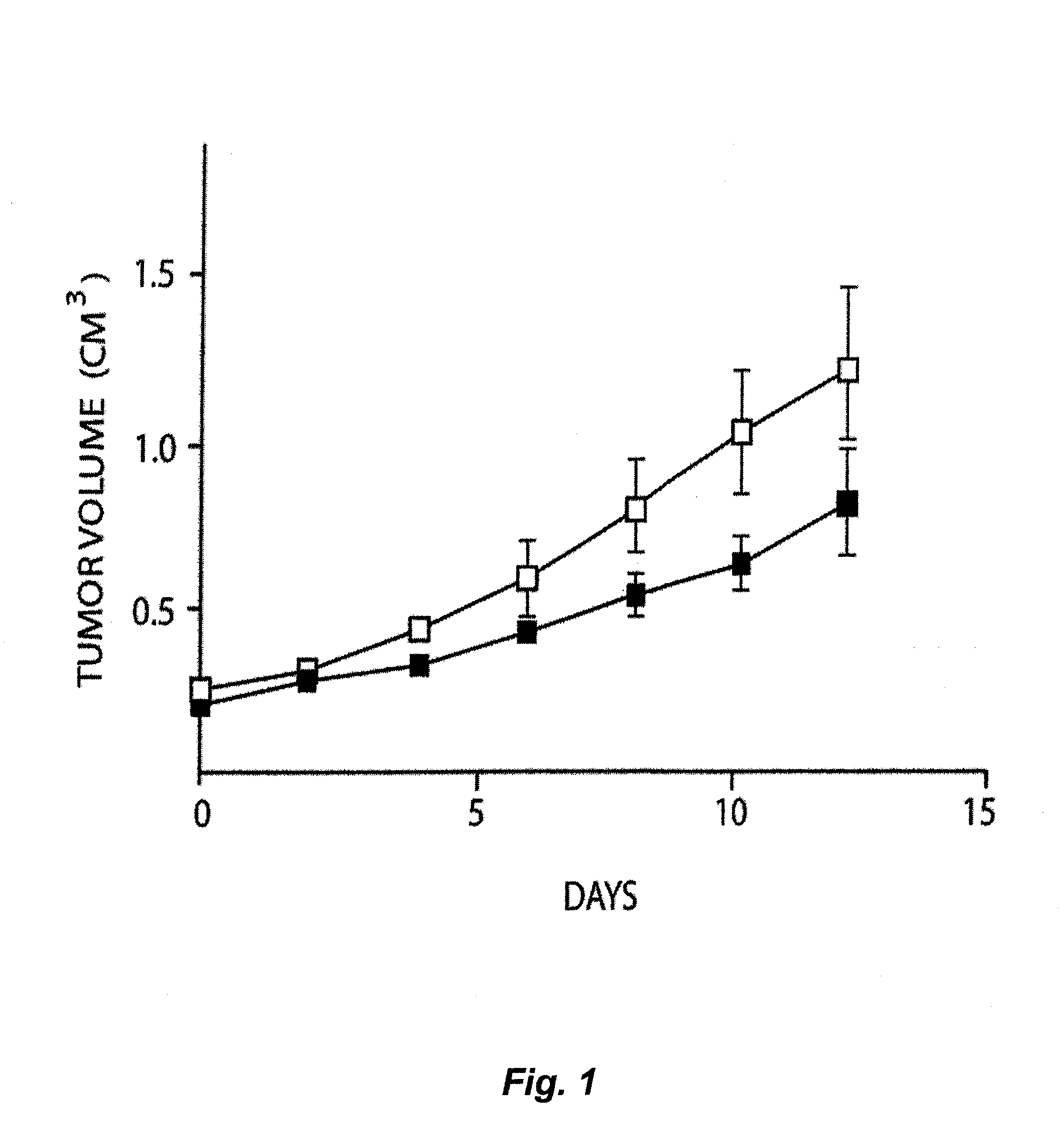
Treatment of Solid Tumours
a solid tumour and treatment technology, applied in the field of solid tumour treatment, can solve the problems of poor penetration into the 3-d tumour, unable to meet the needs of chemotherapy,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
The Compound of the Invention Induces Apoptosis and Reduces Viability of MCS
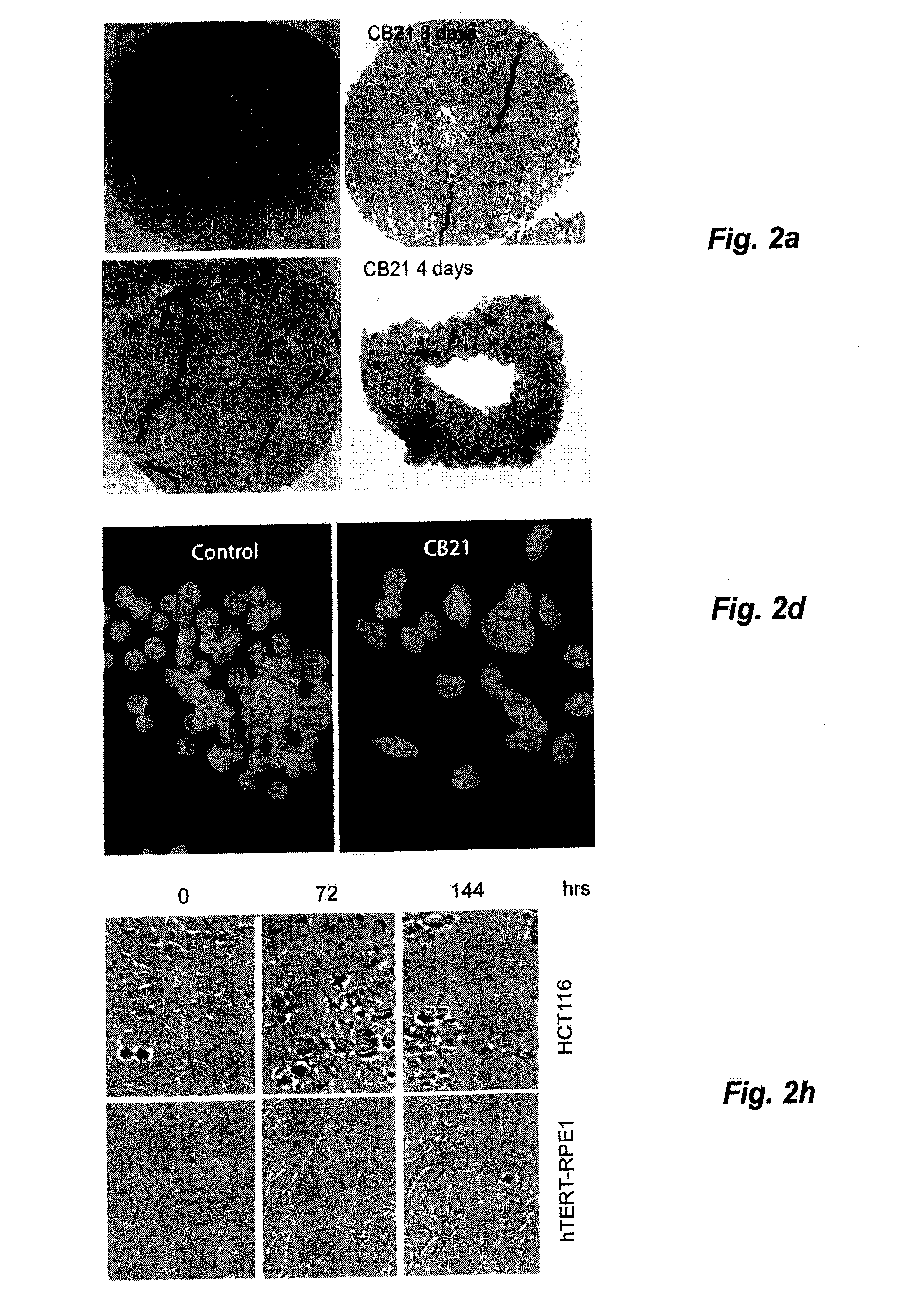
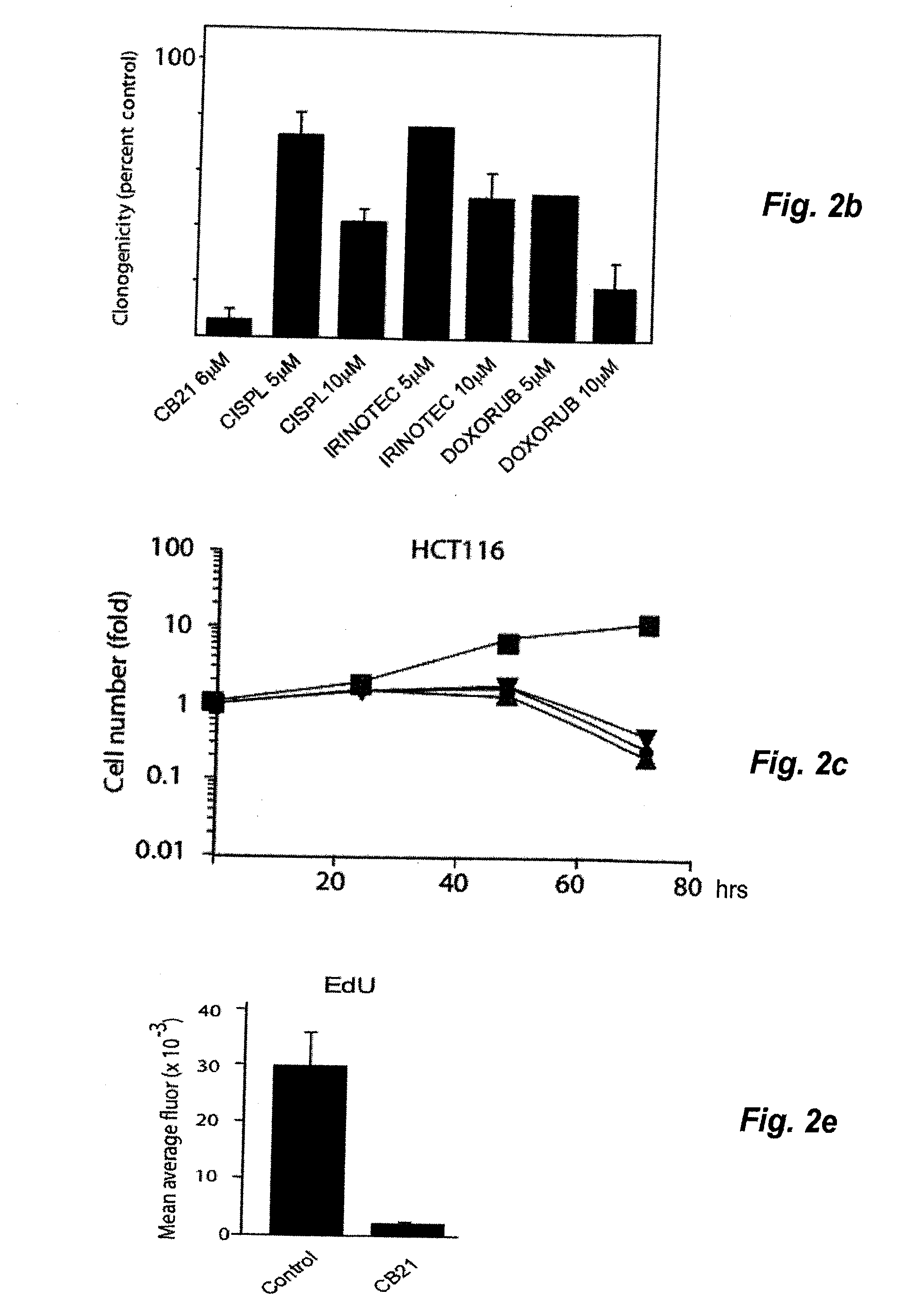
[0045]Treatment of MCS with CB21 for 6 h followed by incubation for 96 h in drug-free medium resulted in MCS of smaller size with central areas of necrosis (FIG. 2a). Caspase-3 induction was modest compared to NSC647889. Importantly, treatment of MCS for 6 hours with CB21 reduced the clonogenicity to b). The decrease in clonogenicity was stronger than that observed for cisplatin, irinotecan and doxorubicin (despite the use of concentrations of 5-10 μM; >10-fold the IC50 of these compounds in monolayer cultures). Treatment of monolayer HCT116 cells with CB21 resulted in a slight increase in cell numbers between 0 to 24 h, followed by loss of cells (FIG. 2c). Examination of 5-ethynyl-2′-deoxyuridine incorporation (EdU) in CB21-treated cells showed that DNA synthesis was almost completely abrogated at 24 hours (FIG. 2d, 2e). CB21 was equally effective on cells where the p53 tumour suppressor gene had been disr...
example 2
The Compound of the Invention is a Cell Permeable Iron Chelator
[0046]To generate hypotheses regarding the mechanism of action of CB21, the Connectivity Map (CMap) (15), a compendium of gene expression signatures from drug-treated cell lines, was used. The changes in gene expression elicited by CB21 were most similar to those of ciclopiroxolamine (CPX), an antimycotic agent with iron chelating capacity (17) (FIG. 3a). To test whether the cytotoxic activity of CB21 was dependent on iron depletion, iron chloride was added to HCT116 cells prior to the addition of CB21. Iron chloride was found to totally abrogate the effect of CB21 (FIG. 3b), both on HCT116 cells expressing wtp53 as on HCT116 cells where the p53 gene has been disrupted.
[0047]The anti-proliferative activity of CB21 was compared with that of other known iron chelators. CB21 was found to be more potent than VLX50, deferasirox, ciclopiroxolamine, deferoxamine (FIG. 3c). Structure-activity relationships were examined by use ...
example 3
The Compound of the Invention Induces a Widespread Autophagic Response
[0048]The anti-tumourigenic activity of iron chelators is generally attributed to inhibition of ribonucleotide reductase, leading to inhibition of cell proliferation (18). MCSs contain mostly non-proliferating cells. The finding of induction of cytotoxic effects on MCSs by the iron chelator CB21 thus was unexpected. The mechanism(s) of action was studied in more detail. Visual inspection of CB21-treated cells revealed that cells contained multiple large cytoplasmic vesicles (FIG. 4a). These vesicles stained positively with an antibody to microtubule associated protein 1 light chain 3 (LC3), suggesting that they were associated with autophagy. LC3 staining was observed at 24 h and was stronger at 42 h (FIG. 4b). Western blot analysis showed that treatment of HCT116 monolayer cells with CB21 induced a strong increase in the levels of both LC3-I and LC3-II (FIG. 4c). LC3-II (the PE-conjugated form of LC3) is a prote...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Permeability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com