Chlorine evolution anode
a technology of chlorine evolution and anode, applied in the field of chlorine evolution anode, can solve the problems of increasing the potential of the anode, increasing the electrolytic voltage, and reducing the service life and durability of the anod
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0052]A commercially available titanium plate (5 cm in length, 1 cm in width, 1 mm in thickness) was immersed and etched in a 10% oxalic acid solution at 90° C. for 60 minutes and then washed and dried. Next, prepared was a coating solution which was obtained by adding ruthenium trichloride trihydrate (RuCl3.3H2O) and tantalum pentachloride (TaCl5) to a butanol (n-C4H9OH) solution containing 6 vol % concentrated hydrochloric acid so that the mole ratio of ruthenium to tantalum is 90:10 and the total of ruthenium and tantalum is 50 g / L in terms of metal. This coating solution was applied to the titanium plate dried as mentioned above, dried at 120° C. for 10 minutes, and then thermally decomposed for 20 minutes in an electric furnace that was held at 260° C. This series of application, drying, and thermal decomposition was repeated five times in total in order to prepare a chlorine evolution anode of Example 1, the anode having a catalytic layer formed on the titanium plate that was ...
example 2
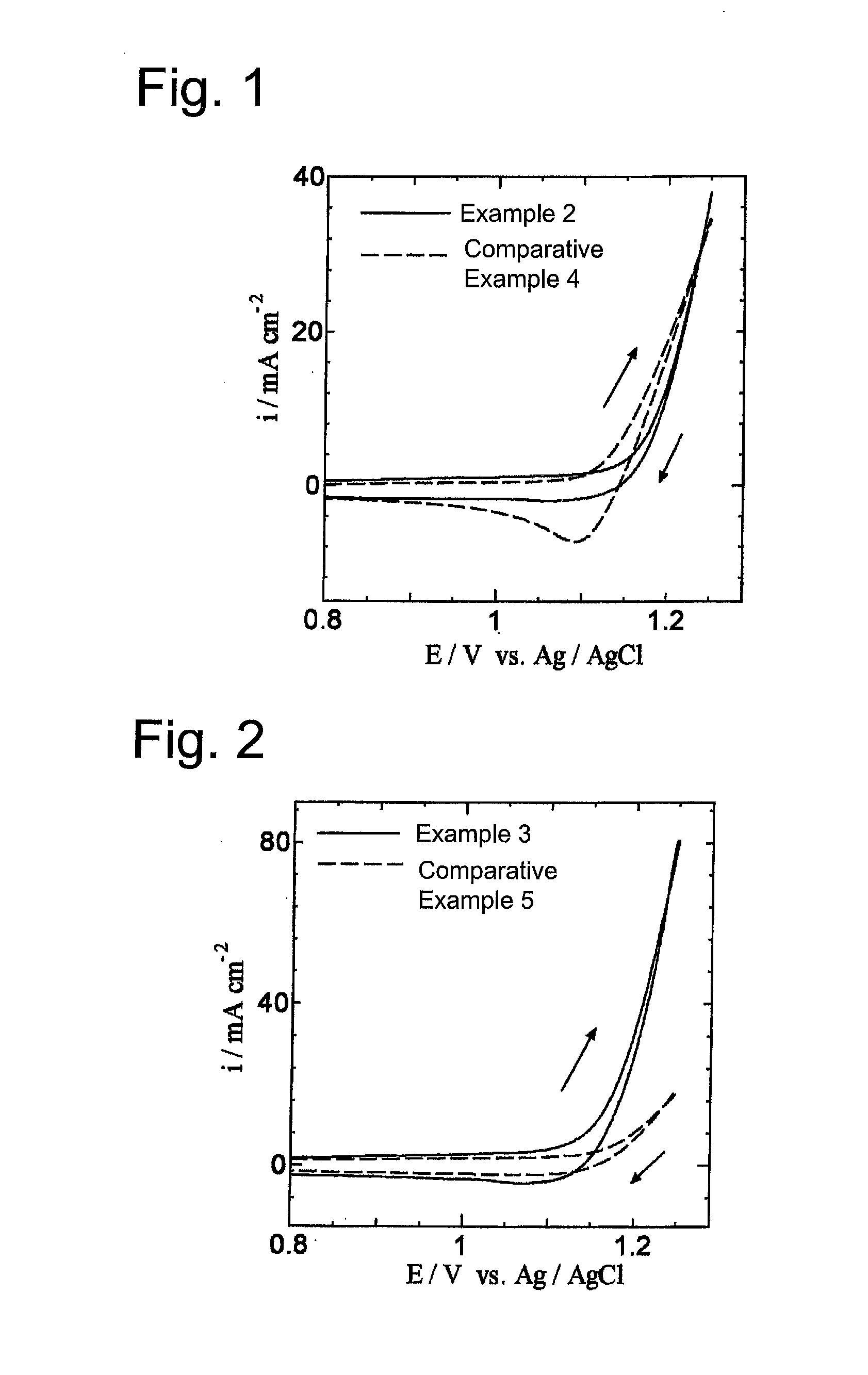
[0064]A commercially available titanium plate (5 cm in length, 1 cm in width, 1 mm in thickness) was immersed and etched in a 10% oxalic acid solution at 90° C. for 60 minutes and then washed and dried. Next, prepared was a coating solution which was obtained by adding ruthenium trichloride trihydrate (RuCl3.3H2O) and tantalum pentachloride (TaCl5) to a butanol (n-C4H9OH) solution containing 6 vol % concentrated hydrochloric acid so that a mole ratio of ruthenium to tantalum was 30:70 and a total of ruthenium and tantalum was 50 g / L in terms of metal. This coating solution was applied to the titanium plate dried as mentioned above, dried at 120° C. for 10 minutes and then, thermally decomposed for 20 minutes in an electric furnace that was held at 280° C. This series of application, drying and thermal decomposition was repeated five times in total in order to prepare a chlorine evolution anode in which a catalytic layer was formed on the titanium plate that was a conductive substrat...
example 3
[0069]The chlorine evolution anode of Example 2 was used to measure cyclic voltammograms under the same conditions except that the cobalt electrowinning solution of Example 2 was changed to a hydrochloric acid electrolytic solution, the pH of which was adjusted to 1.6 by adding only hydrochloric acid to distilled water and the scan rate was changed to 50 mV / s.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Electric energy | aaaaa | aaaaa |
| Electrical conductivity | aaaaa | aaaaa |
| Electrical conductor | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com