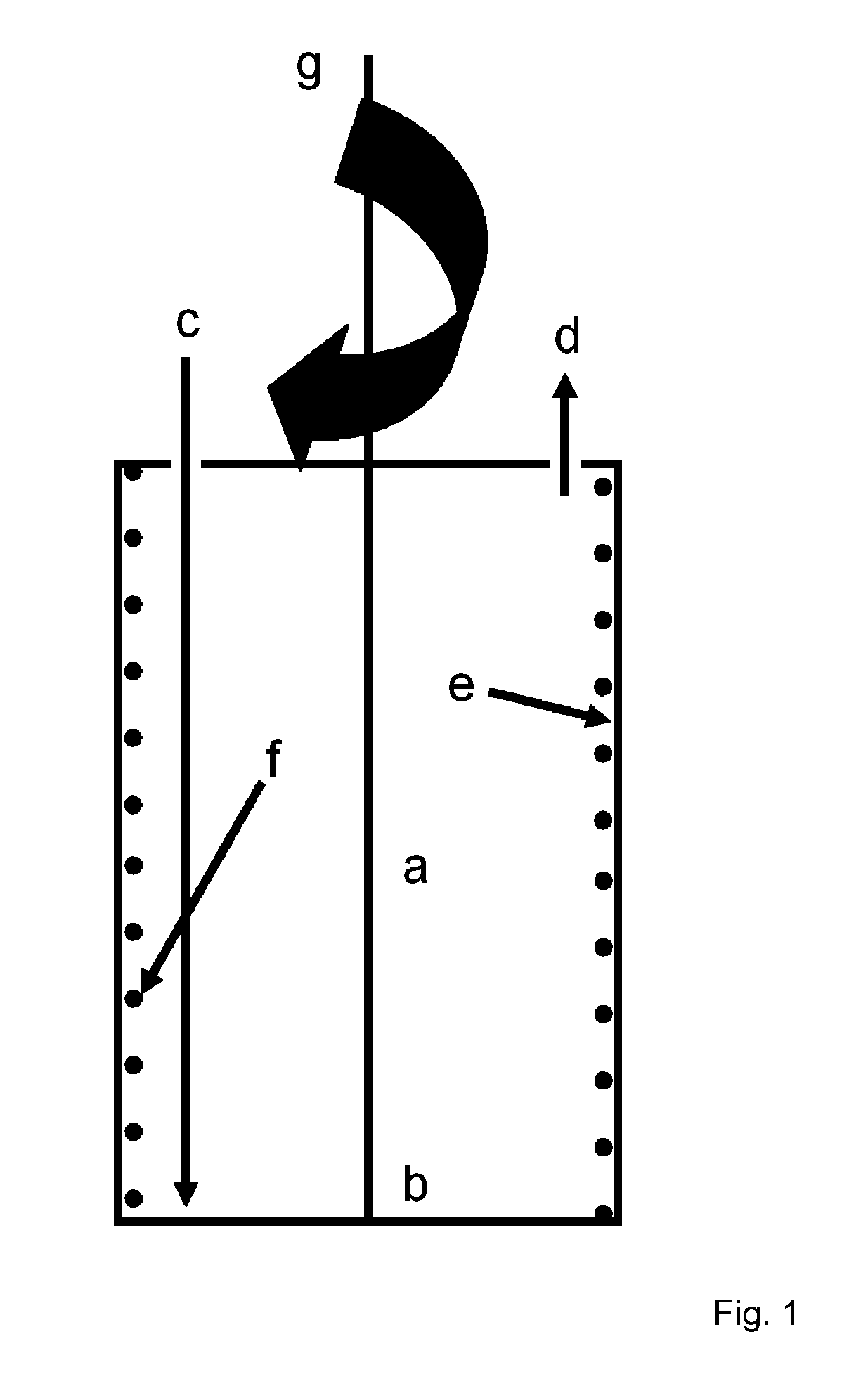
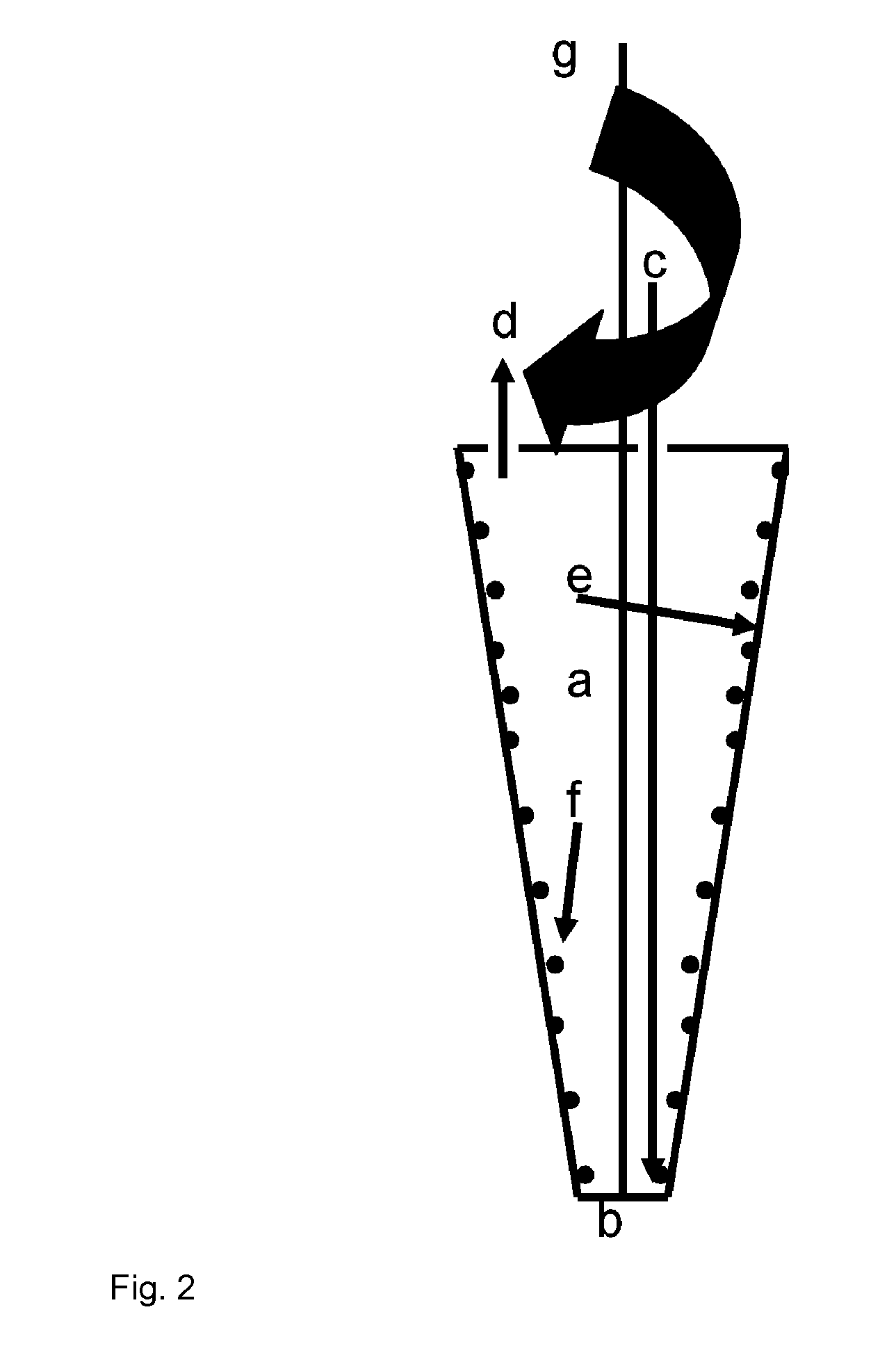
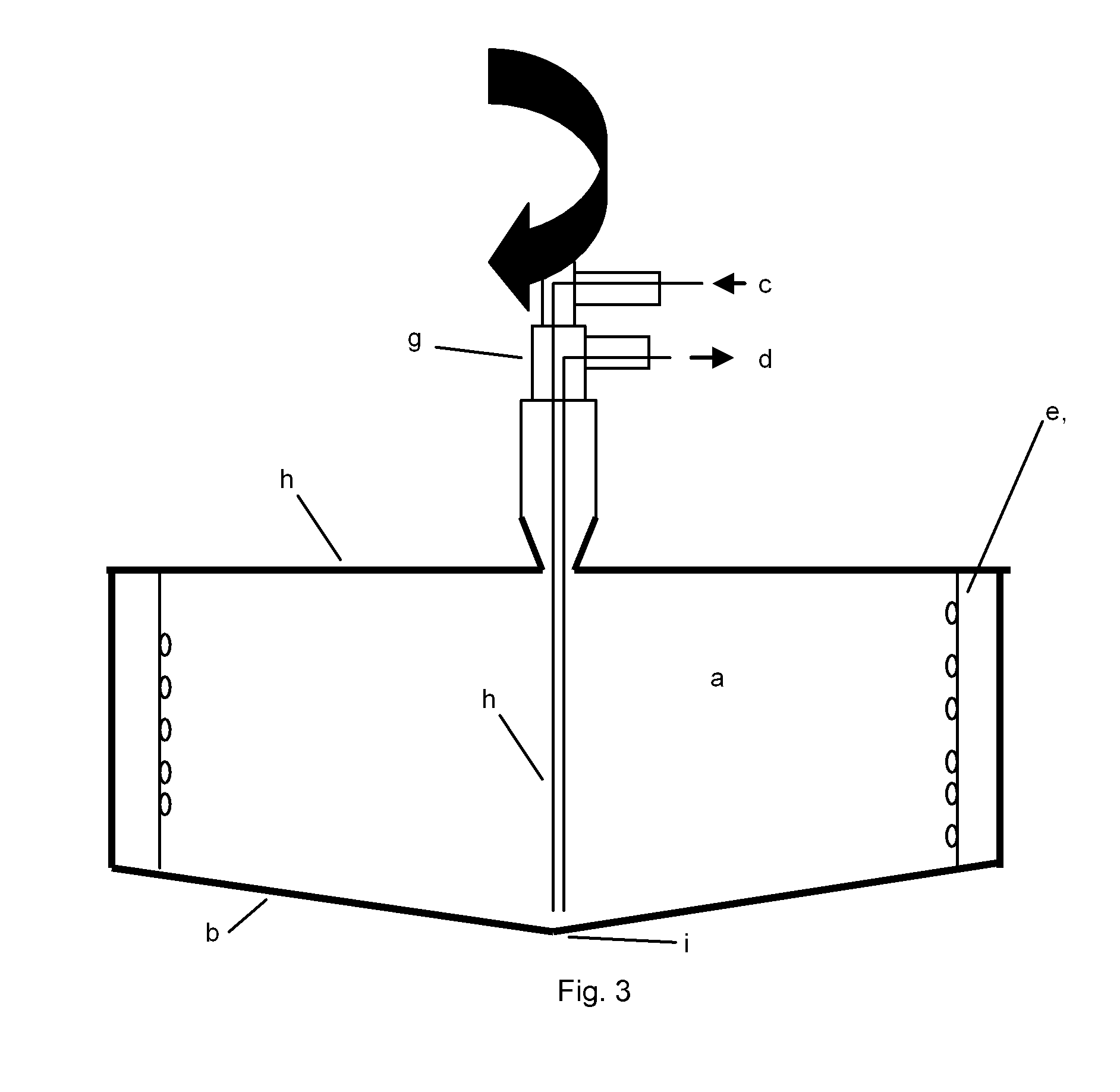
Method and device for cell modification
a cell modification and cell technology, applied in the field of cell modification, can solve the problems of difficult culturing large numbers of cells adhered difficult to achieve culturing large numbers of cells to adhere to a surface without the use of difficult to achieve culturing large numbers of cells to adhere to a surface without the use of carriers or large volume cell suspensions, etc., to achieve speed up the development o
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Viral Transduction of T Cells with Disease-Specific T Cell Receptor Genes
[0167]A use of the invention is the introduction of genes coding for a disease-specific T cell receptor into a polyclonal population of T cells, which may then be used for therapeutic injection into patients. The T cells are directed towards the target antigen, e.g. tumor cell or infected cells.
[0168]A centrifugation chamber providing cell modifying surfaces coated with RetroNectin® is supplied with a recombinant virus containing supernatant, wherein the virus encodes the target antigen, and rotated at surfaces by the gravitational forces generated by the rotation. Following this coating step, the chamber is rotated at low rotation speed and the T cells to be modified are introduced into the high rotational speed (e.g. 2000×g) for 2 hours. For improved viral transduction, the T cells are previously activated, e.g. by cultivation in the presence of antibodies against CD3 and CD28, either in the same centrifugati...
example 2
Activation and Expansion of Antigen-Specific T Cells
[0171]T cells can be activated and expanded by antigens loaded in or on antigen-presenting cells (APC). T cell activation requires intimate contact between the T cells and APC.
[0172]To improve T cell activation a system described herein is used to spin down APC and T cells in an appropriate ratio, e.g. 1:100 to 100:1. Either physiological cell mixtures such as PBMC, containing T cell and APC or defined cell preparations, e.g. purified T cells and APC, e.g. dendritic cells, B cells, macrophages, cell lines transfected with distinct MHC molecules, etc, mixed at an appropriate ratio are used. In addition antigens, proteins, peptides, cell lysates, and growth factors and / or co-stimulatory antibodies, e.g. anti CD28, antiCD137, may be added. The contact between the cells is rapidly induced and maintained at an appropriate level by centrifugation.
[0173]APC and T cells can be deposited in distinct layers, e.g. T cell on top of a layer of ...
example 3
Polyclonal Activation and Expansion of T Cells
[0176]The systems of the invention provide an optimized platform for polyclonal activation and expansion of T cells, comprising conventional T cells or regulatory T cells.
[0177]This example is similar to Example 2 except that instead of defined antigen, polyclonal stimuli are used, comprising antibodies against CD3 and co-stimulatory molecules, such as CD28 and / or CD137. These antibodies are added either in soluble form, requiring the addition of accessory cells bearing Fc-receptors, e.g. conventional antigen-presenting cells or cell lines transfected with Fc-receptors. Alternatively the added antibodies are immobilised on a macroscopic surface, e.g. a particle or bead ranging from about 30 nm to 100 μm. These immobilised antibodies are directly cultured with purified T cells, e.g. at ratios 1:4 to 4:1. As described above the system used allows regulated contact of T cells and stimulating agent and controlled addition of additional envir...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Angle | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Force | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com