Cancer targeting using carbonic anhydrase isoform ix inhibitors
a carbonic anhydrase and inhibitor technology, applied in the direction of biocide, organic chemistry, drug composition, etc., can solve the problems of ca ix, poor radiotherapy response, metastatic formation, etc., to reduce the extracellular acidosis of tumors, reduce the risk of ca ix, and improve the effect of radiosensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
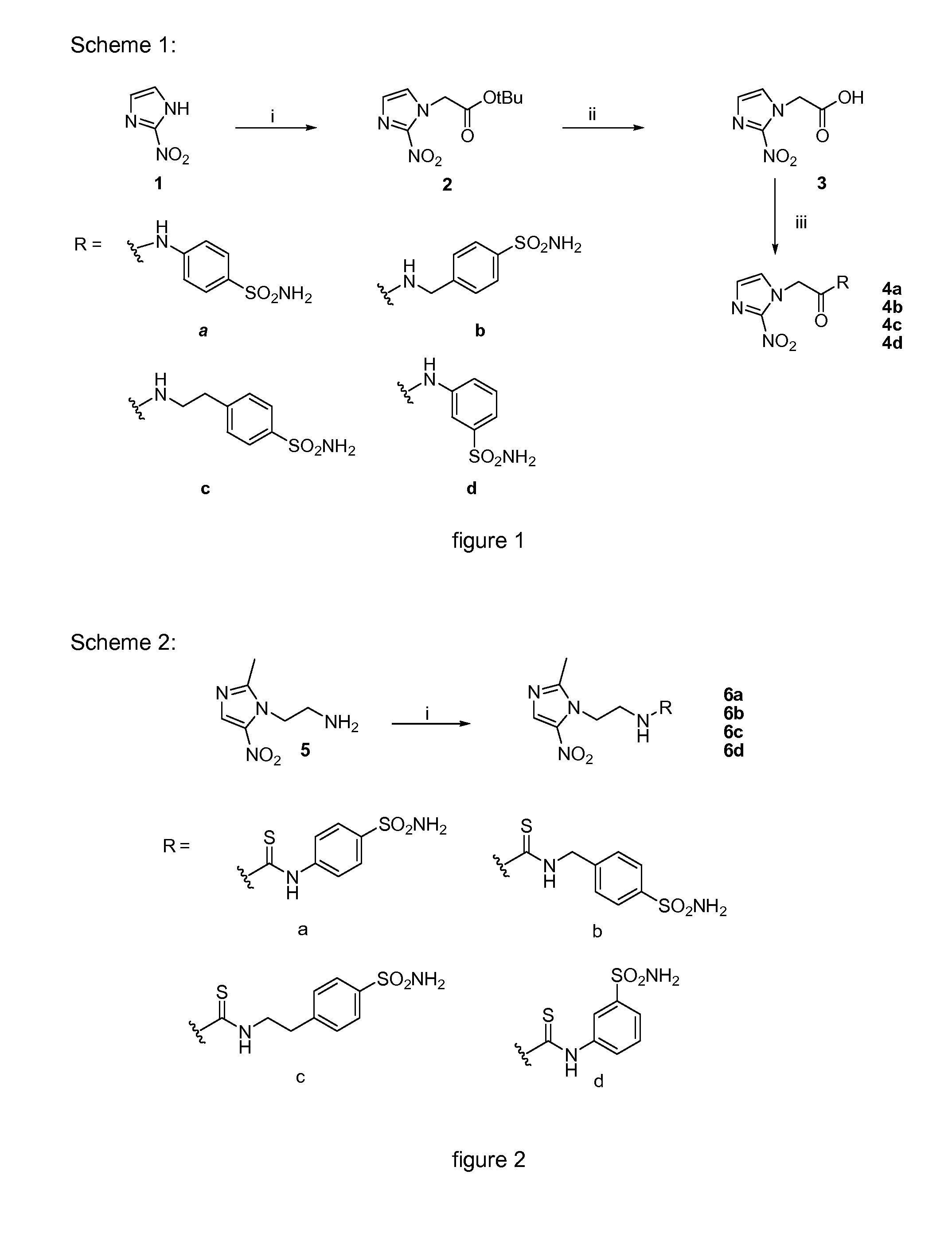
[0034]FIG. 1 shows scheme 1.
[0035]Reagents and conditions: (i) 1 equiv. of 2-nitroimidazole, 1 equiv. of tert-butyl bromoacetate, 4 equiv. of potassium carbonate, MeCN, RT, 1 night; (ii) cocktail of trifluoroacetic acid / water / thioanisole 95 / 2.5 / 2.5 v / v, room temperature, 1 night; (iii) 1 equiv. of 4-dimethylaminopyridine (DMAP), 1 equiv. of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC), N,N-dimethylacetamide (DMA), room temperature, 2 days.
[0036]FIG. 2 shows scheme 2.
[0037]Reagents and conditions: (i) 1 equiv. of 1-(2-aminoethyl)-2-methyl-5-nitroimidazole dihydrochloride monohydrate, 1 equiv. SCN-Ph-SO2NH2, 2 equiv. of triethylamine, MeCN, room temperature, 1 hour.
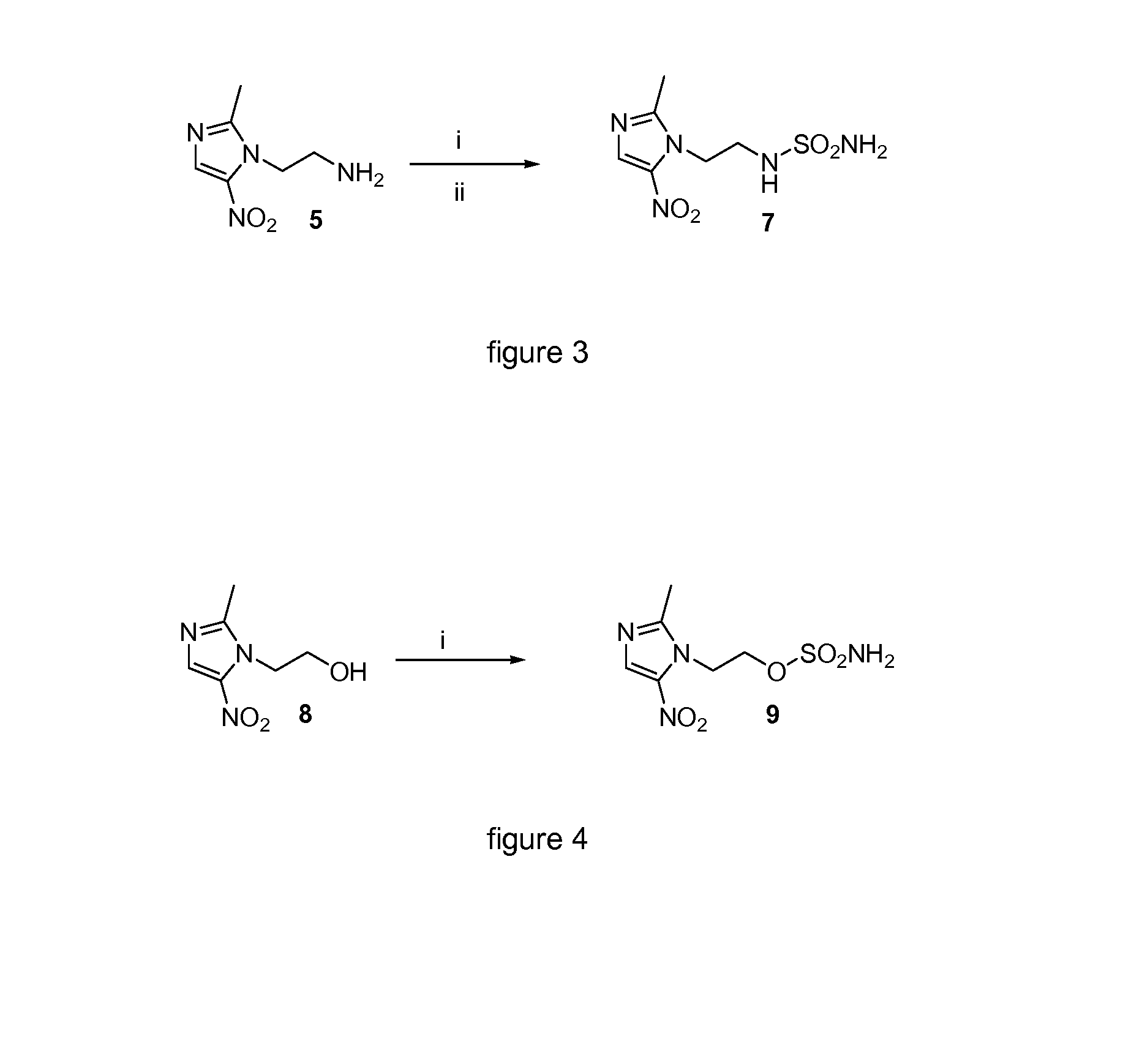
[0038]FIG. 3 shows the scheme of the preferred compound of the invention.
[0039]Reagents and conditions: (i) 1 equiv. of 1-(2-aminoethyl)-2-methyl-5-nitroimidazole dihydrochloride monohydrate, 4 equiv. of triethylamine, 1 equiv. of chlorosulfonylisocyanate, 1 equiv. of tert-butanol, CH2Cl2, rt, 1 hour; (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| humidity | aaaaa | aaaaa |
| humidity | aaaaa | aaaaa |
| humidity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com