New stable salt of 5,10-methylene-(6R)-tetrahydrofolic acid
a technology of tetrahydrofolic acid and hemisulfate salt, which is applied in the direction of biocide, drug composition, inorganic non-active ingredients, etc., can solve the problems of inability to predict the existence of a stable solid (polymorphic) form of a (known) chemical compound with suitable properties, cell death and delay of tumor growth,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of (6R)-5,10-CH2-THF hemisulfate salt
[0062]A solution of (6S)-tetrahydrofolic acid (16 mmol, 7.93 g) in 78.0 g distilled water was provided in a roundbottom flask at room temperature under N2. The pH of this solution was adjusted to pH 11 by adding (slowly) a 32% NaOH solution. As soon as the solution became clear, a 1.00M HCl solution was added to adjust the pH of the solution to 8.3 at 25° C. The obtained clear solution was cooled to about 0° C., at which temperature it showed a pH of 8.8. By addition of 1M HCl the pH was adjusted to pH=8.6 and 1.44 g of a 36.8% HCHO solution (110 mol %) were added in one portion. Upon completion of the addition the solution was stirred at 0° C. (ice bath) for 1 hour. Active charcoal (0.2 g, Norit C Extra) was added and the reaction mixture was stirred for 30 minutes at 0° C. and then cold filtered over a suction filter to obtain a clear solution, which was used in step (b) without further purification.
[0063](b) A mixture of 55 ml 1M H...
example 2
Characterization
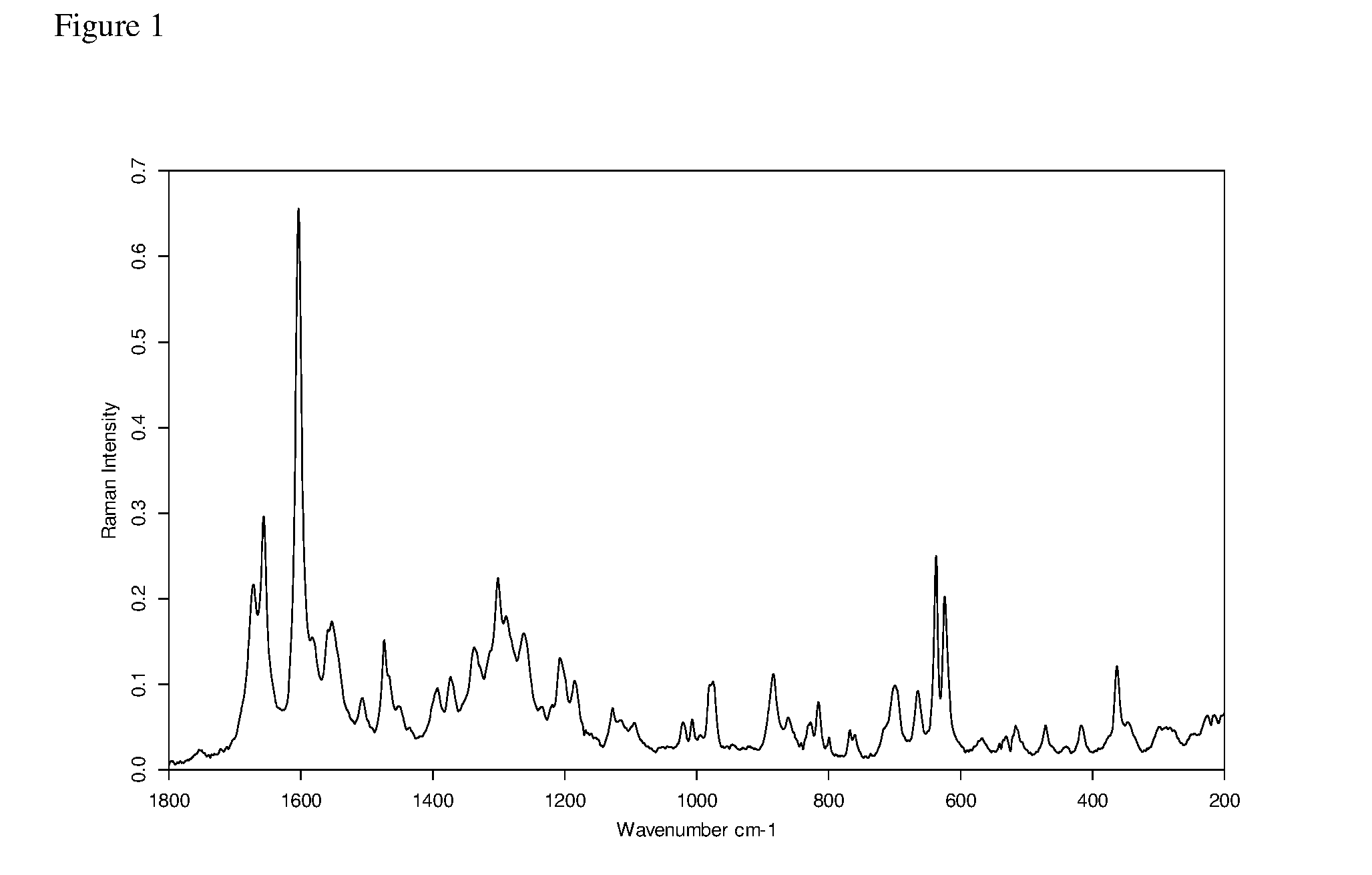
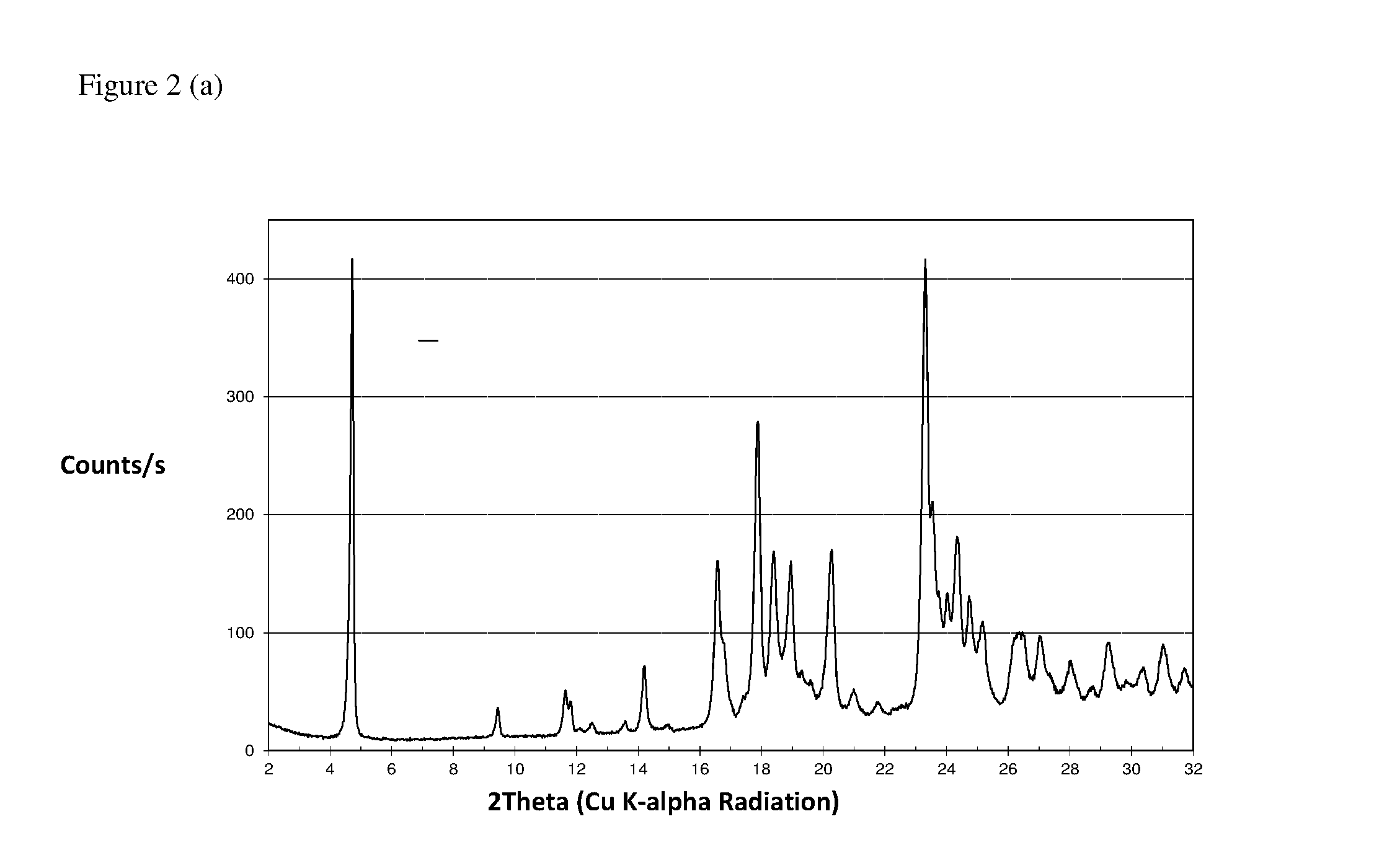
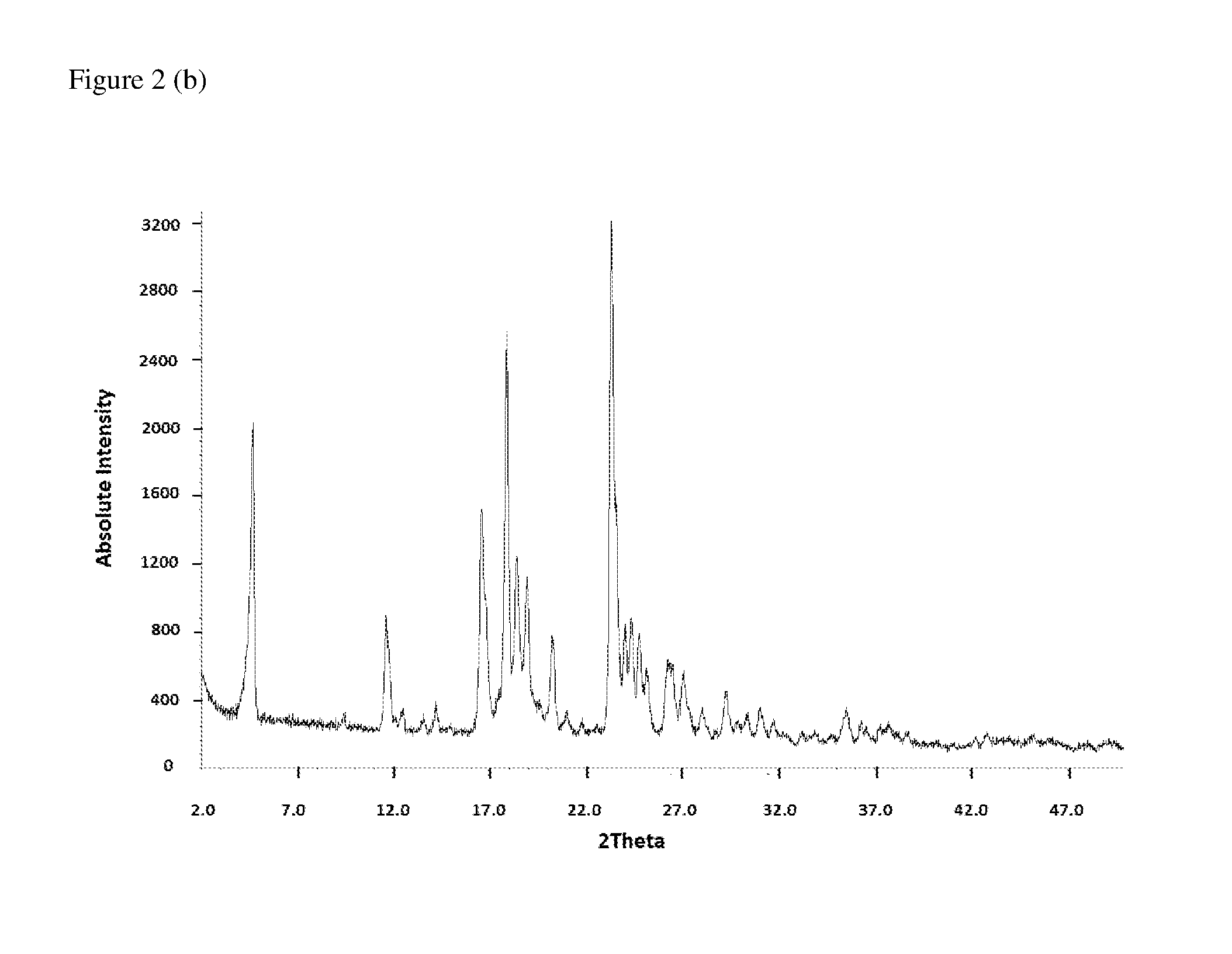
[0064](a) The FT Raman spectrum of (6R)-5,10-CH2-THF hemisulfate salt, recorded using a nominal laser power level of 300 mW and 64 scans is shown in FIG. 1.[0065](b) The corresponding powder X-ray diffractogram, recorded in transmission mode, is shown in FIG. 2.[0066](c) The TG-FTIR thermogram of (6R)-5,10-CH2-THF hemisulfate salt is shown in FIG. 3. It was carried out under N2 flow (to avoid oxidative degradation). The sample shows a loss of 0.5 wt % H2O from ca. 40° C. to 210° C., which is residual water (due to either hygroscopicity or incomplete drying). Decomposition occurs only above 210° C.[0067](d) The DSC thermogram of (6R)-5,10-CH2-THF hemisulfate salt is shown in FIG. 4. Prior to the first heating scan, the sample was equilibrated for three minutes under gaseous nitrogen flow and lost 0.6 wt.-% of its mass during that time. This is consistent with the water content observed in the TG-FTIR thermogram (see FIG. 3) and confirms that this water is loosely boun...
example 3
Stability Testing of (6R)-5,10-CH2-THF hemisulfate salt
[0072](a) Suspension equilibration of (6R)-5,10-CH2-THF hemisulfate salt as starting material at temperatures other than room temperature in a variety of solvents and mixture are summarized in Table 10:
TABLE 10Suspension equilibration stability of(6R)-5,10-CH2-THF hemisulfate saltTemper-Durationature(h: hours;Observa-Solvent(s)(° C.)d: days)tionMeOH / formic acid 1:1502 hNo changeAcOH saturated with L-ascorbic acid501 dNo changeTHF with ~2 mM L-ascorbic acid403 dNo change2-PrOH with ~2 mM L-ascorbic acid403 dNo changePEG4500 / EtOH 1:9 saturated with507 dNo changeL-ascorbic acidH2O 56 dNo changeformic acid / THF 1:310-206 dNo changeAcOH saturated with L-ascorbic acid505 dNo changeMeCN saturated with L-ascorbic acid505 dNo change
[0073](b) Stability in 85% ethanol at room temperature
[0074](6R)-5,10-CH2-THF hemisulfate salt (3.01 g) was dispersed in 100 ml 85% EtOH at room temperature and stirred for 5 h, then filtered and dried at 30° C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com