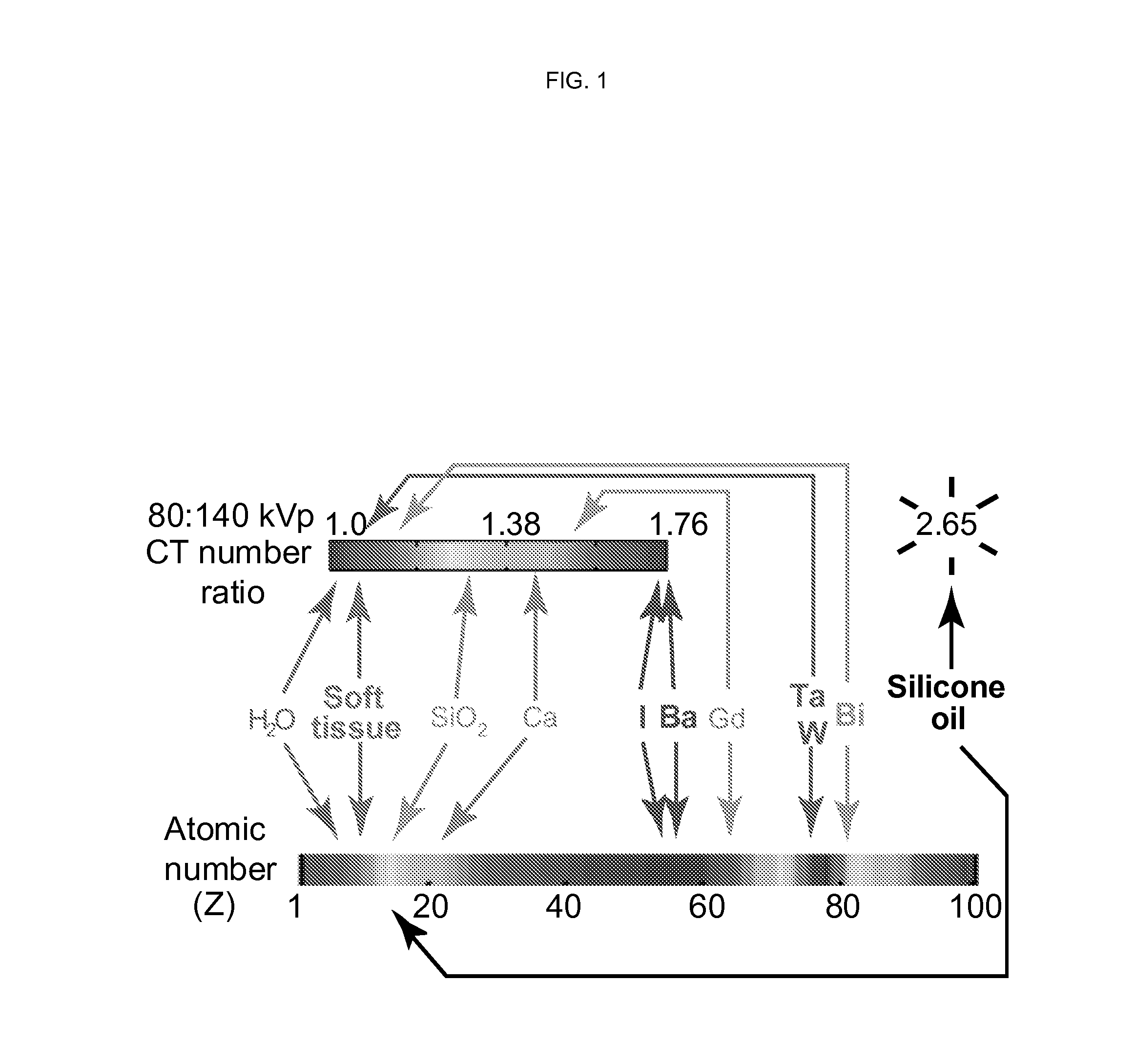
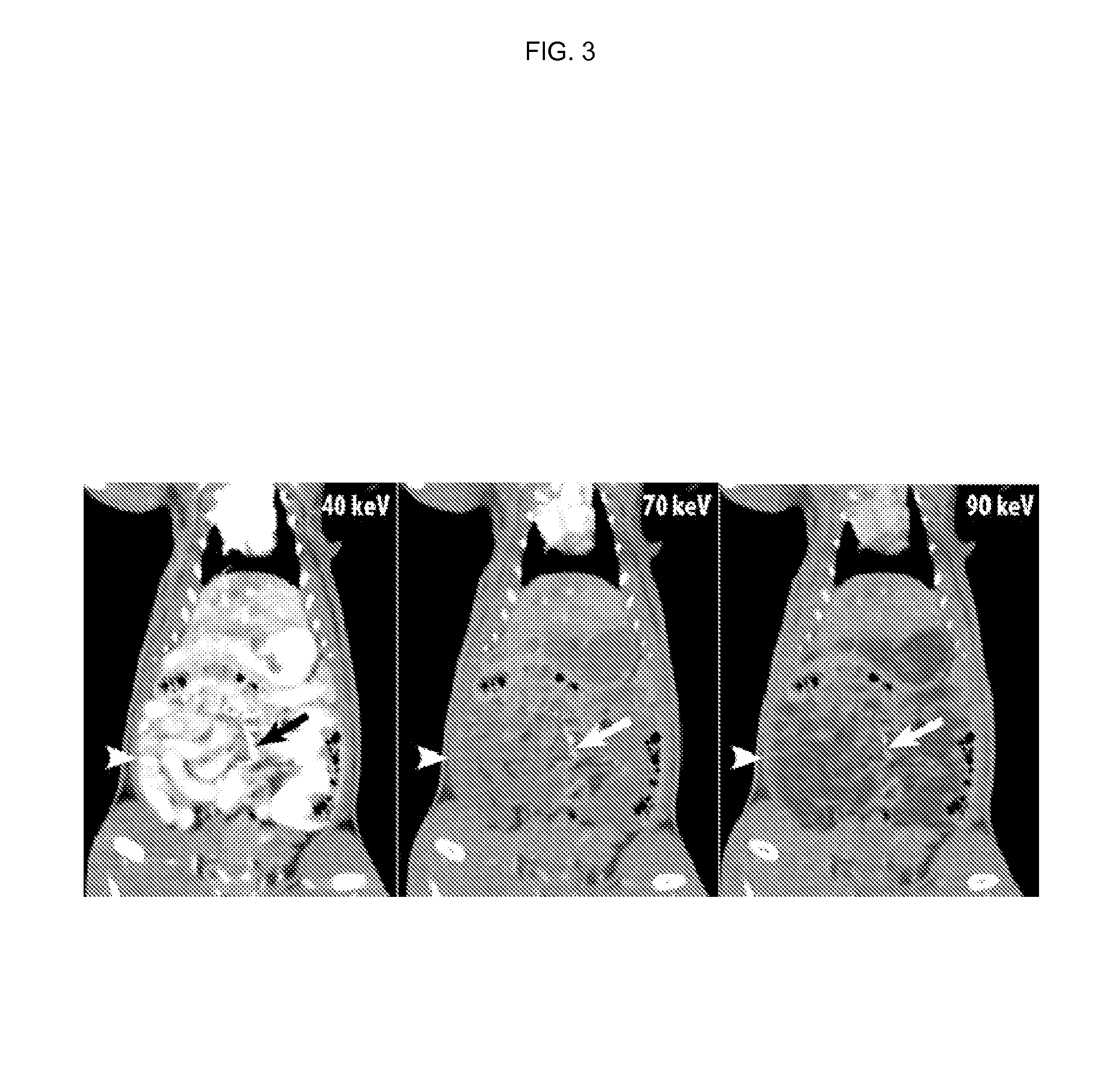
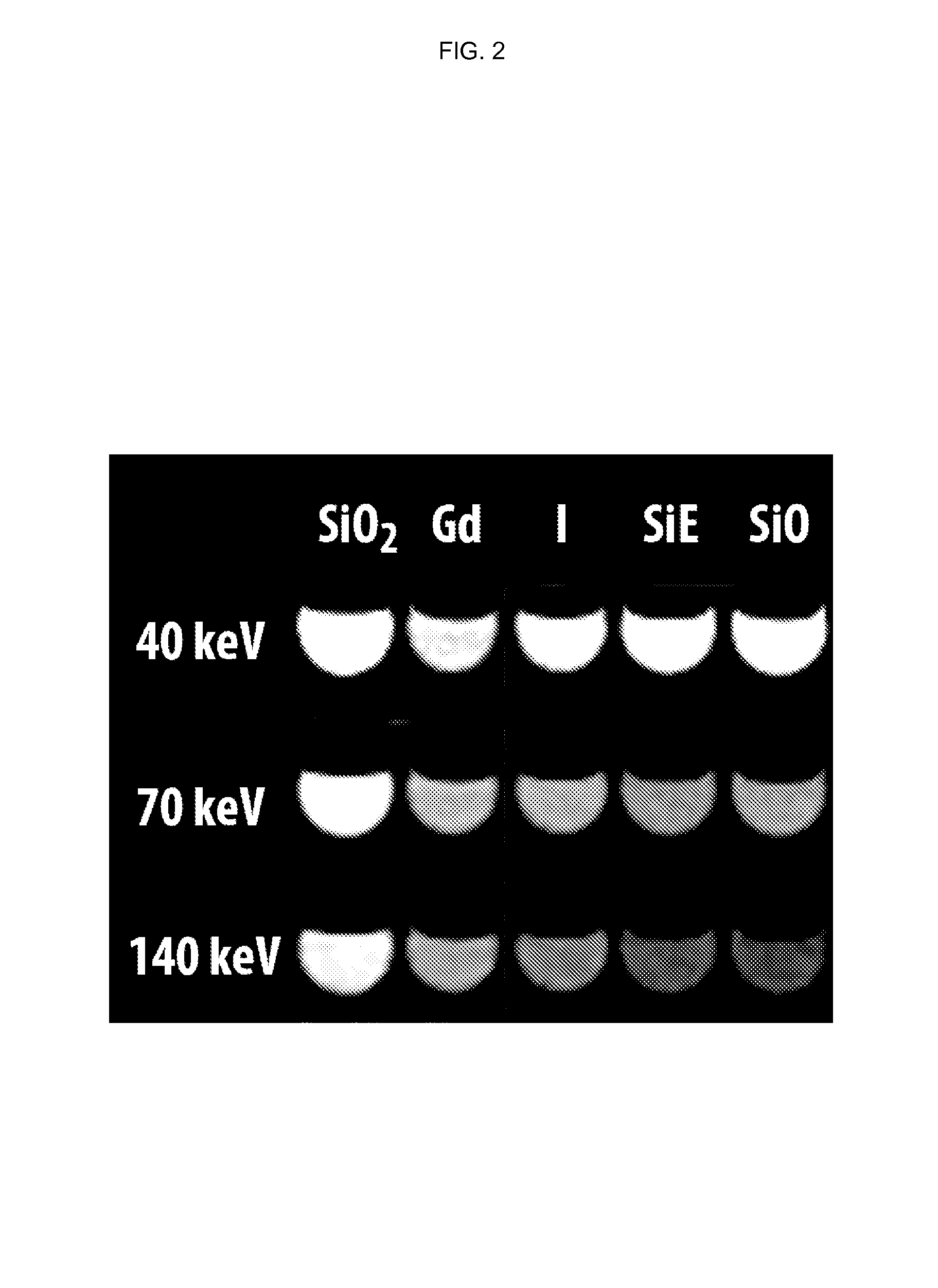Silicone-based enteric CT contrast material
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0186](Method 1) One percent of surfactant Tween 20 (Sigma-Aldrich) aqueous solution was prepared by dissolving 50 mg of Tween-20 in 4.95 g of distilled water. To this clear solution (in a 50-ml centrifuge tube) was added dropwise 15.0 g of silicon-based polymer (50 cSt@ 25° C., Fisher Scientific) during moderate vortexing, through a 20-ml syringe attached with a 23G needle. After completion of the addition, the mixture was vigorously vortexed at room temperature for 3 minutes, giving a 75 wt % oil-in-water (o / w) type emulsion with 0.25wt % of Tween 20 surfactant (Note: tiny air bubbles are not avoidable in non-vacuum preparation conditions). This emulsion was measured to give the average particle size as 60 microns on a Malvern 3000 ZetaSizer. The emulsion can be stably stored at room temperature without phase separation for at least 3 months. CT scan proved its homogeneity (except the existence of tiny air bubbles).
[0187](Method 2) One percent of surfactant Tween-20 (Sigma-Aldrich...
example 2
[0190]The procedure of Example 1 was followed using Triton X-100.
example 3
[0191]The procedure of Example 1 was followed using Triton X-100 and a fluorinated silicon-based polymer FS-1265 (viscosity 350 cSt, Dow Corning).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com