Compositions and methods for increasing mesenchymal stromal cell migration to tumors
a mesenchymal stromal cell and tumor technology, applied in the field of compositions and methods for increasing the migration of mesenchymal stromal cells to tumors, can solve the problems of only being able to apply to a minority of patients, and the mechanisms involved in the recruitment of mscs to tumors in general, and achieve the effect of increasing the migration of mscs and increasing the adhesion of mscs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods
Cell Lines
[0092]Human HCC cell line HuH7 were kindly provided by Prof. Jesus Prieto (CIMA, University of Navarra, Pamplona, Spain). LX-2 cell line (human HSCs generated by spontaneous immortalization in low serum conditions) was kindly provided by Dr. Scott Friedman (Division of Liver Diseases, Mount Sinai School of Medicine, New York, N.Y., USA). Human microvascular endothelial cells (HMEC-1) were provided by CDC (Centers for Disease Control, Atlanta, Ga., USA). Cell lines were cultured in complete DMEM (2 μmol / L glutamine, 100 U / mL penicillin, 100 mg / mL streptomycin and 10% heat-inactivated fetal bovine serum (FBS)). Primary culture of HCC cells (HC-PT-5) was previously generated in our laboratory and cultured the eight passage in 70% DMEM / 30% F12 (Invitrogen / Life Technologies) culture medium supplemented with 2 μmol / L glutamine, 100 units / mL penicillin, 100 mg / mL streptomycin and 10% FBS.
Isolation of MSCs, AT-MSCs and Mesenchymal Cells Harvested from Umbilica...
example 2
Identification of Secreted Factors from HCC Microenvironment
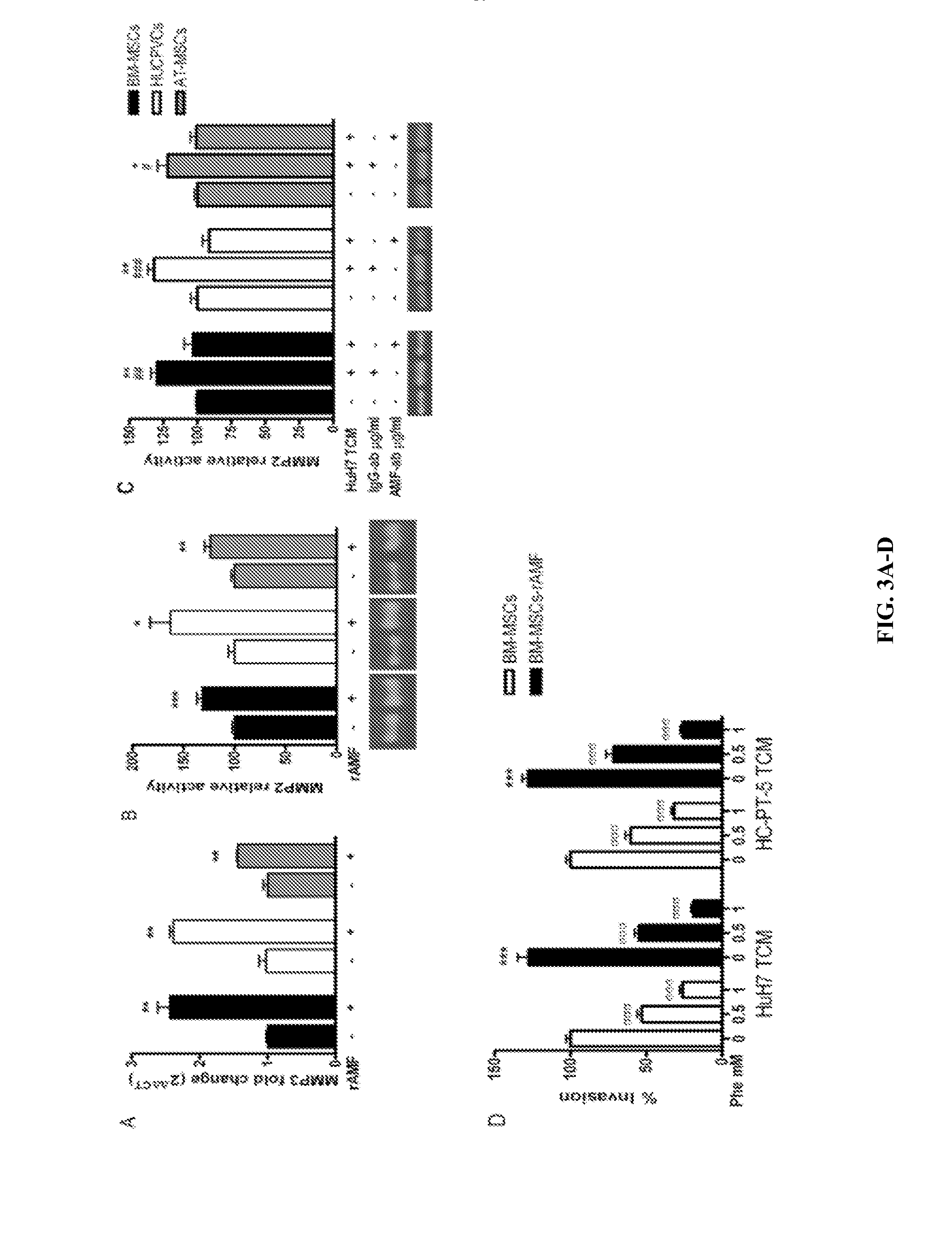
[0111]Factors secreted from hepatocellular carcinoma (HCC) microenvironment were identified. Tumor conditioned media (TCM) were obtained from fresh HCC samples or tumors generated from primary cultured human HCC cells (HC-PT-5) or the HuH7 cell line in BALB / c nude mice.
[0112]In vitro migratory capacity of MSCs to different TCM samples was analyzed using a 48-Transwell microchemotaxis Boyden Chamber unit (Neuroprobe, Inc.).
[0113]Factors present in the different TCM were identified using two Human Cytokine and Chemokine Antibody Arrays (RayBiotech). The factors identified are shown in Tables 1 and 2.
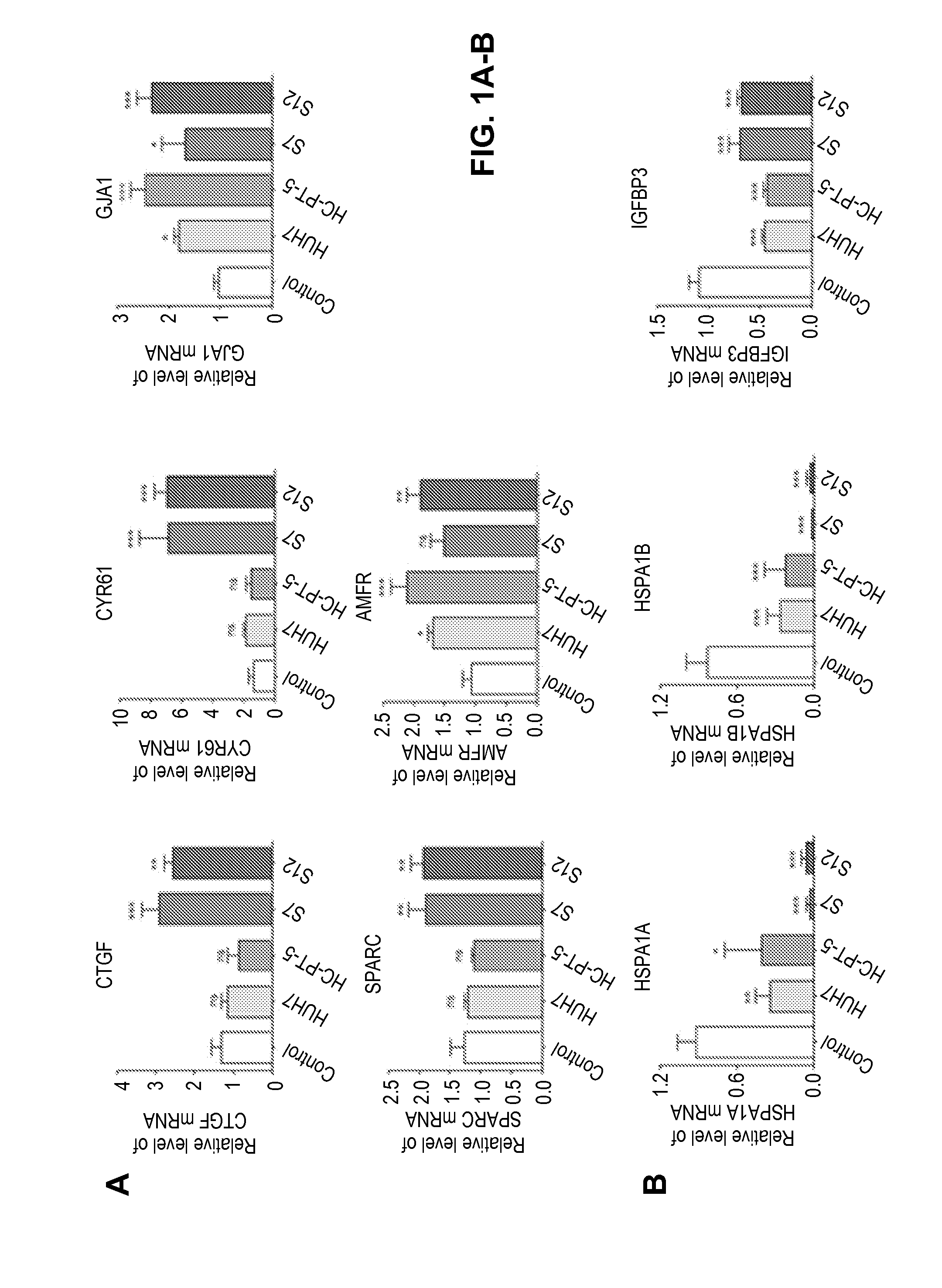
[0114]Changes in the gene expression patterns in MSCs exposed to TCM derived from HCC samples were also analyzed. MSCs were exposed overnight to TCM or DMEM (as control) and studied using a microarray gene expression analysis with the aim to identify genes that were differentially expressed in MSCs exposed or not to TCM. Table 3 s...
example 3
Recombinant AMF Exerts a Specific Chemoatractant Activity on MSCs from Different Sources
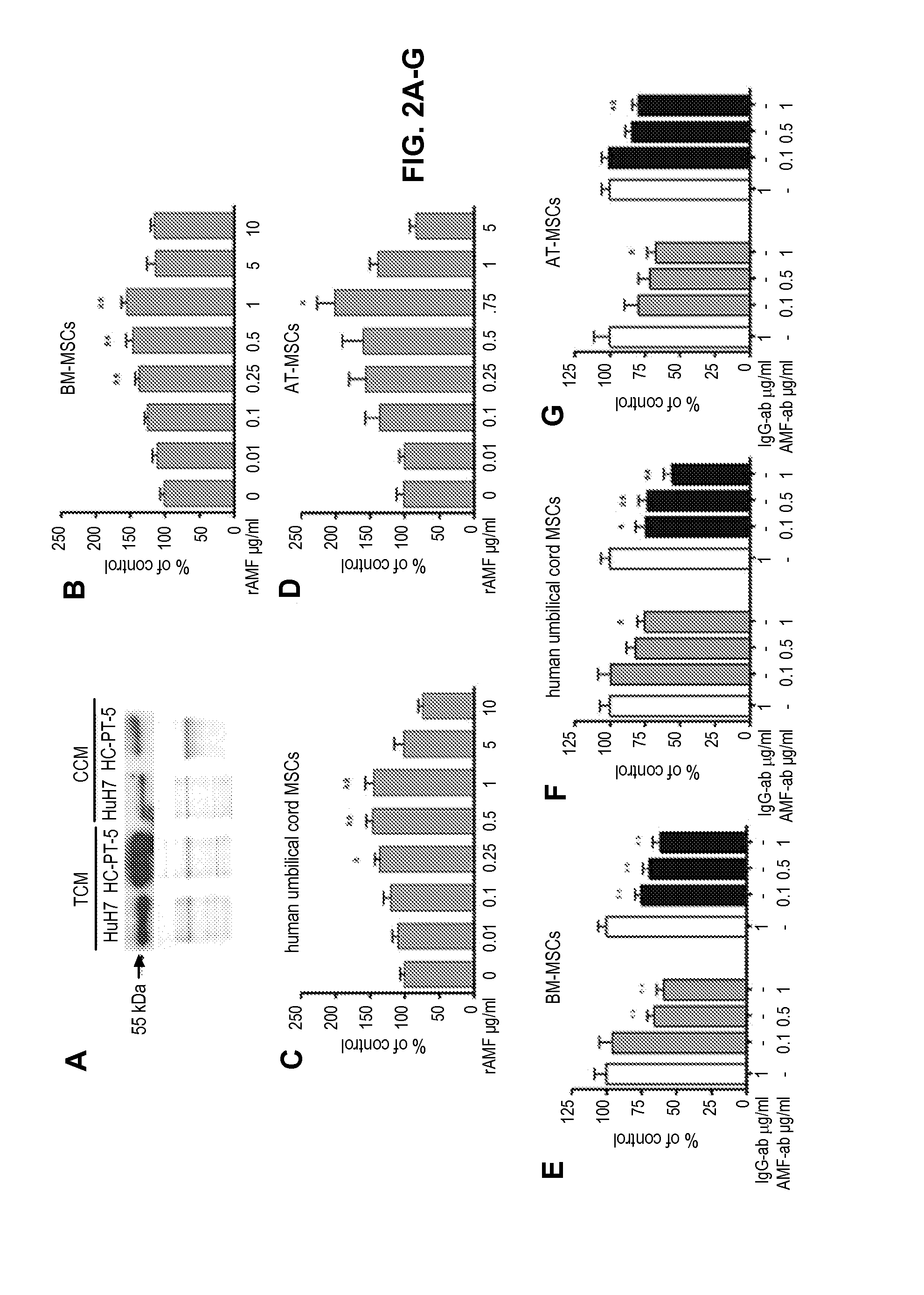
[0117]The tumor conditioned media (TCM) from ex vivo subcutaneous (s.c.) tumors derived from HuH7 cell line or HC-PT-5 HCC primary culture and conditioned media from cell culture monolayers (CCM) were subjected to western blot analysis according to the method described in Example 1. A 55 kDa soluble AMF was detected in CCM and TCM (FIG. 2A).
[0118]The ability of recombinant human AMF (rAMF) to induce MSCs chemotaxis in vitro was analyzed. MSCs from different sources were evaluated by in vitro migration assay with modified Boyden chambers as described in Example 1. Human MSCs derived from bone marrow (BM-MSCs), perivascular umbilical cord region (Mesenchymal cells harvested from umbilical cord perivascular tissue), or adipose tissue (AT-MSCs) were used in a modified Boyden chamber assay.
[0119]The MSCs from the different sources migrated in a dose-dependent manner towards recombinant AMF (FIG. 2B-D)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com