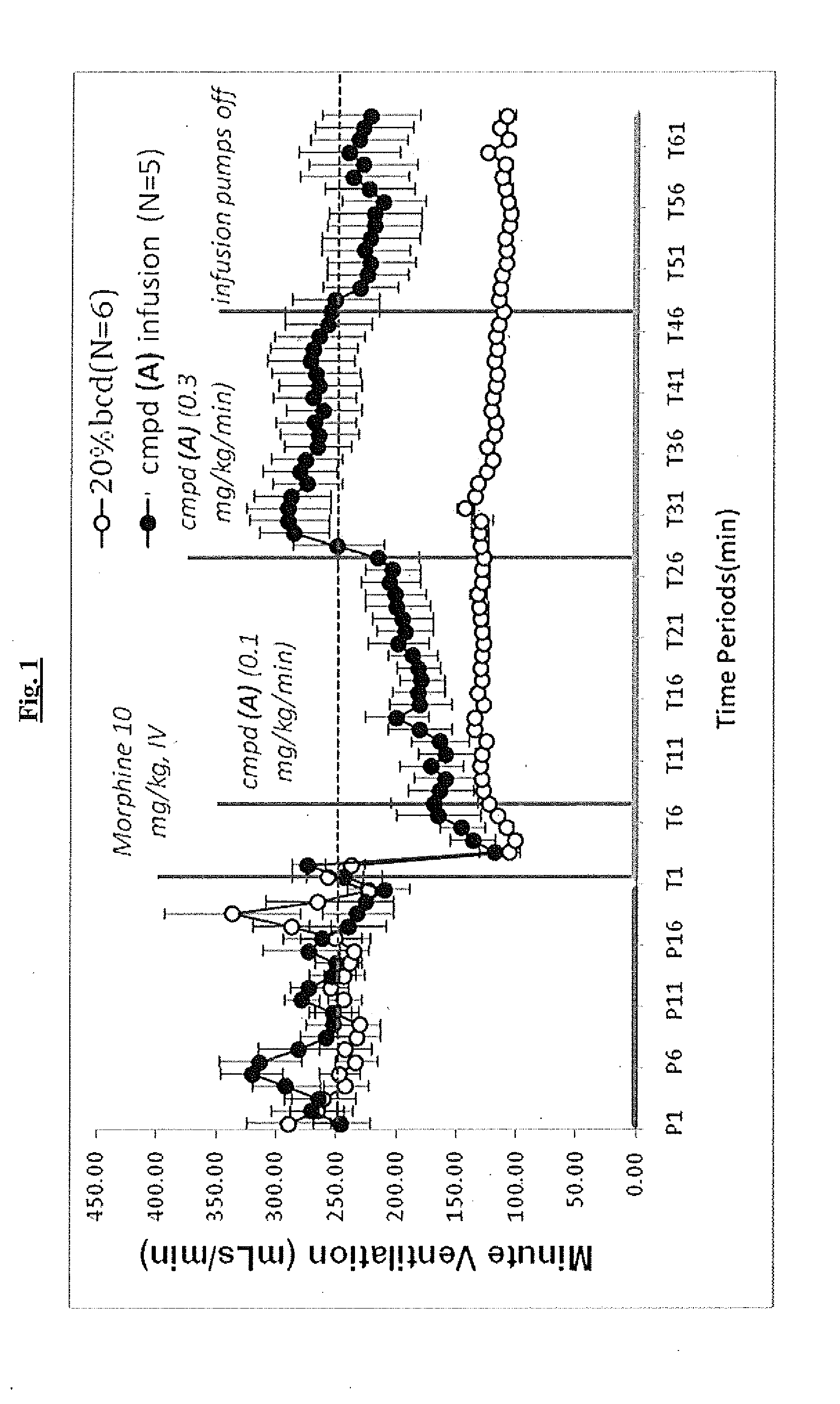
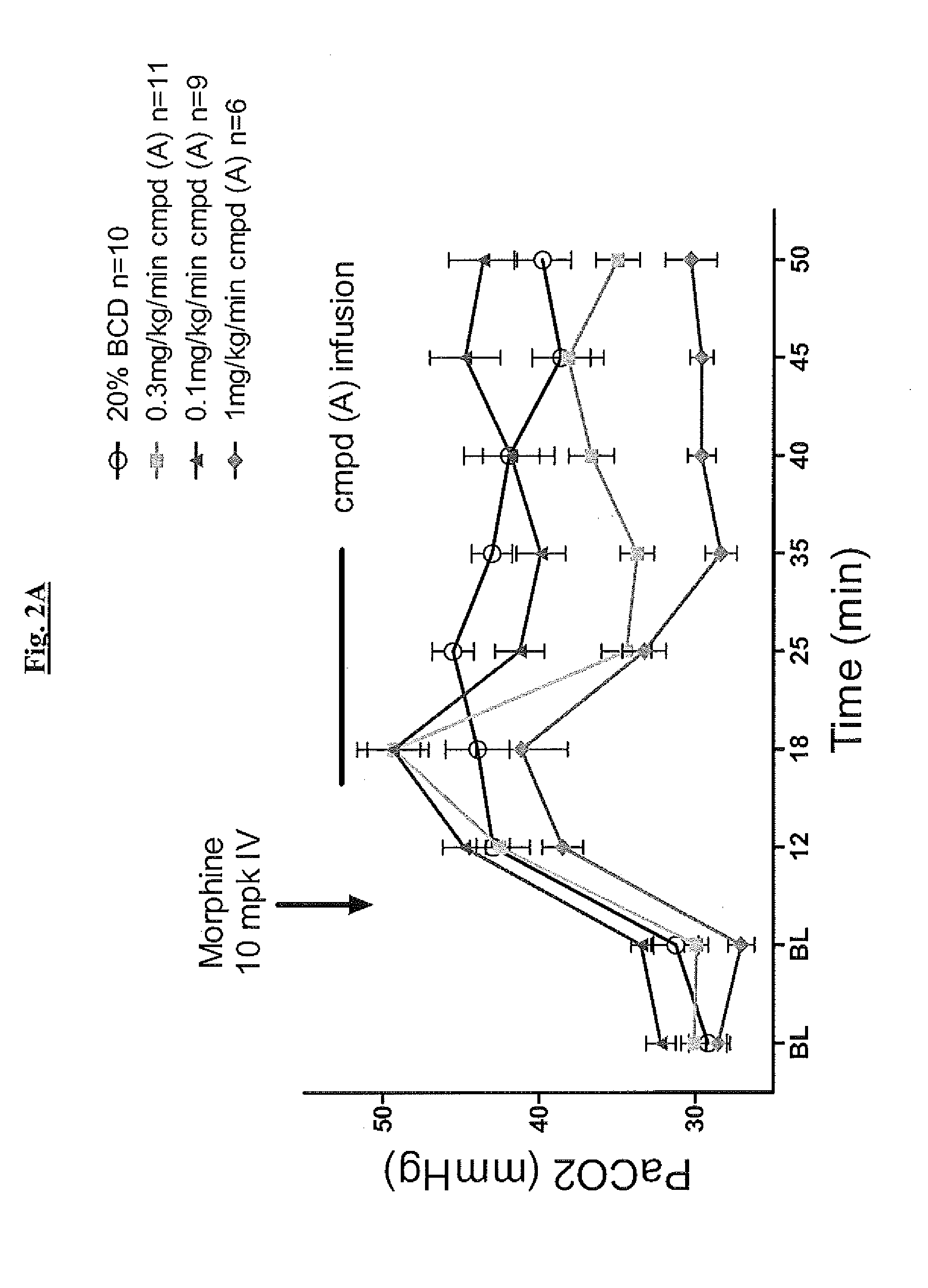
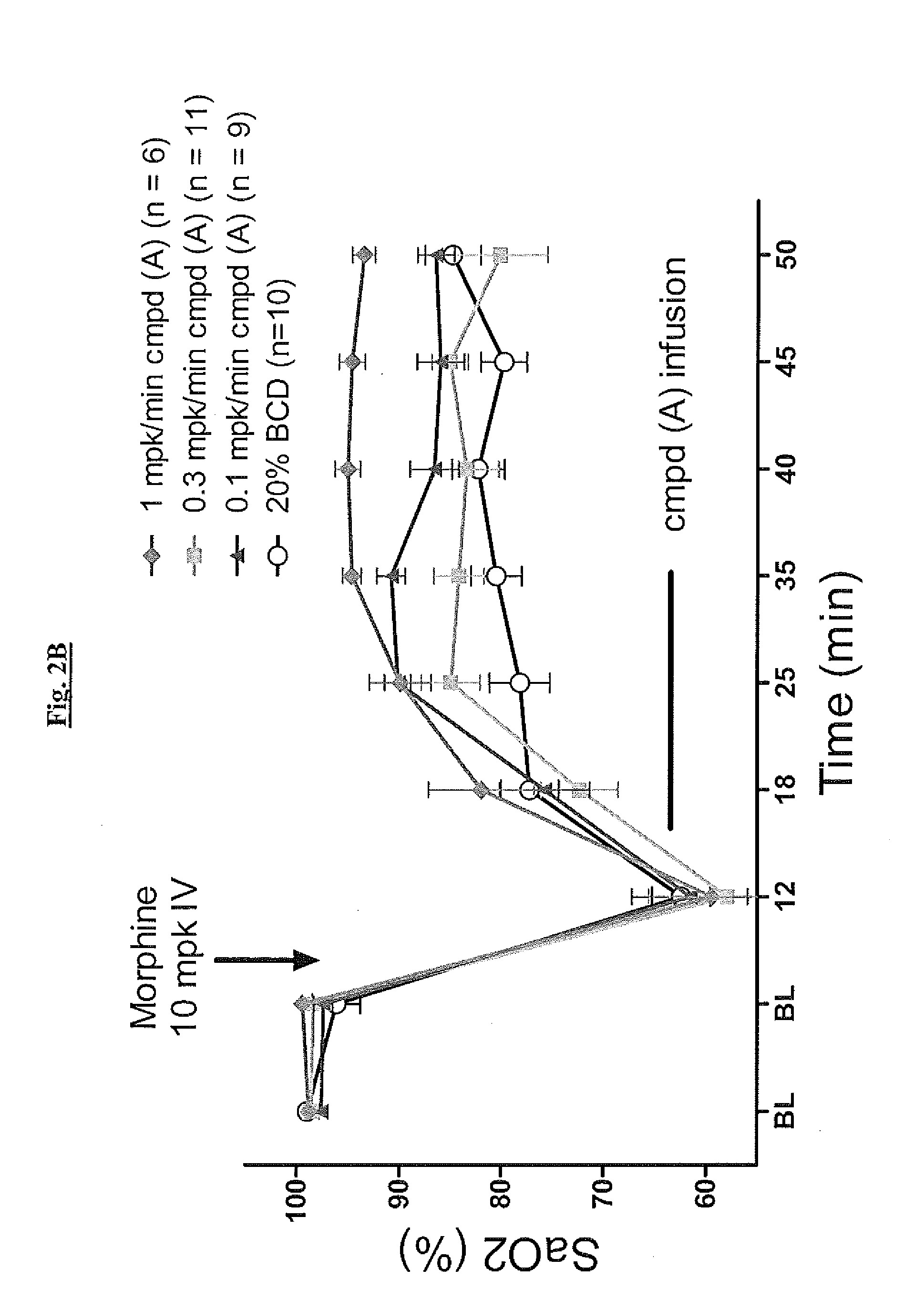
Novel compounds as respiratory stimulants for treatment of breathing control disorders or diseases
a technology of breathing control and compounds, applied in the direction of respirators, drug compositions, extracellular fluid disorders, etc., can solve the problems of severe cardiovascular consequences, hypoxia (and the associated oxidative stress), and no breathing periods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
N-(4,6-Bis-methylamino-[1,3,5]triazin-2-yl)-N,O-dimethyl-hydroxylamine hydrochloride (XX)
[0245]
2-Chloro-N-(4,6-bis-methylamino)-[1,3,5]triazine (XIX)
[0246]2,4,6-Trichloro-1,3,5-triazine (XVIII) (5.0 g, 27 mmol) was dissolved in acetone (35 mL) and poured into ice-water (50 mL) to form a very fine suspension. A solution of N-methylamine hydrochloride (3.66 g, 54 mmol) in water (20 mL) was added and the temperature maintained at approximately 0° C. To this mixture, 2N NaOH (54 mL, 108 mmol) was added in a dropwise manner to keep the temperature between 0° C. and 5° C. The mixture was stirred 30 min at ambient temperature for an additional 60 min at 50° C. The precipitate was filtered and washed with water (3×25 mL). After drying over anhydrous calcium chloride under high vacuum, 2-chloro-N-(4,6-bis-methylamino)-[1,3,5]triazine (XIX) was isolated as a white powder (4.2 g, 89% yield). LCMS (ESI) m / z=174 (M+H)+.
N-(4,6-Bis-methylamino-[1,3,5]triazin-2-yl)-N,O-dimethyl-hydroxylamine hydroc...
example 2
N-(4,6-Bis-ethylamino-[1,3,5]triazin-2-yl)-N,O-dimethyl-hydroxylamine hydrochloride (XXII)
[0248]
2-Chloro-N-(4,6-bis-ethylamino)-[1,3,5]triazine (XXI)
[0249]2,4,6-Trichloro-1,3,5-triazine (XVIII) (5.0 g, 27 mmol) was dissolved in acetone (35 mL) and poured into ice-water (50 mL) to form a very fine suspension. A solution of ethylamine (2.43 g, 54 mmol) in water (20 mL) was added and the temperature maintained at approximately 0° C. To this mixture, 2N NaOH (27 mL, 54 mmol) was added in a dropwise manner to keep the temperature between 0° C. and 5° C. The mixture was stirred for 30 min at ambient temperature, and for additional 60 min at 50° C. The precipitate was filtered off, washed with water (3×25 mL). After drying over anhydrous calcium chloride under high vacuum, 2-chloro-N-(4, 6-bis-ethylamino)-[1,3,5]triazine (XXI) was isolated as a white powder (5.0 g, 92% yield). LCMS (ESI) m / z=202 (M+H)+.
N-(4,6-Bis-ethylamino-[1,3,5]triazin-2-yl)-N,O-dimethyl-hydroxylamine hydrochloride (XXI...
example 3
N-(4-Cyclopropylmethylamino)-N-(6-n-propylamino) [1,3,5]triazin-2-yl)-N,O-dimethyl-hydroxylamine (XXV)
[0251]
2, 4-Dichloro-N-(6-n-propylamino)-[1,3,5]triazine (XXIII)
[0252]2,4,6-Trichloro-1,3,5-triazine (XVIII) (20 g, 109 mmol) was dissolved in acetone (100 mL) and poured into ice-water (50 mL) to form a very fine suspension. A solution of propan-1-amine (7.1 g, 120 mmol) in water (20 mL) was added and the temperature maintained at approximately 0° C. To this mixture, 2N NaOH (60 mL, 120 mmol) was added in a dropwise manner to keep the temperature between −5° C. and 0° C. The mixture was stirred at 0° C. for 60 min. The precipitate was filtered off and washed with water (3×25 mL). After drying over calcium chloride under high vacuum, 2,4-dichloro-N-(6-n-propylamino)-[1,3,5]triazine (XXIII) was isolated as a white powder (18 g, 80% yield). LCMS (ESI) m / z=208 (M+H)+.
2-Chloro-N-(4-cyclopropylmethyl)-N-(6-n-propylamino) [1,3,5]triazine (XXIV)
[0253]2,4-Dichloro-N-(6-n-propylamino)-[1,3,5]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com