Transmucosal drug delivery devices for use in chronic pain relief
a technology of drug delivery device and chronic pain, which is applied in the direction of pharmaceutical active ingredients, medical preparations, organic active ingredients, etc., can solve the problems of marked alteration in behavior, excessive use of medication and medical services, and restriction in daily activities, so as to efficiently treat chronic pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of the Devices of the Invention
[0064]Transmucosal devices are configured in the form of a disc, rectangular in shape with round corners, yellow on one or both sides of the cheek). Buprenorphine is present in the mucoadhesive layer, and this side is to be placed in contact with the buccal mucosa (inside the cheek). The drug is delivered into and across the mucosa as the disc erodes in the mouth. The non-adhesive, backing layer controls the rate erosion of the disc, and minimizes the amount of buprenorphine dissolved in saliva and ultimately swallowed, a pathway of lower absorption due first pass metabolism. The mucoadhesive polymeric diffusion layer and the backing layer are bonded together and do not delaminate during or after application.
[0065]The mucoadhesive layer for the transmucosal devices of the present invention comprising the desired dosage of buprenorphine is prepared by mixing purified water, propylene glycol (about 4.6% total formulation, by dry weight), sodi...
example 2
Placebo-Controlled, Double-Blind Study to Evaluate the Efficacy of BEMA Buprenorphine in Subjects with Moderate to Severe Chronic Low Back Pain
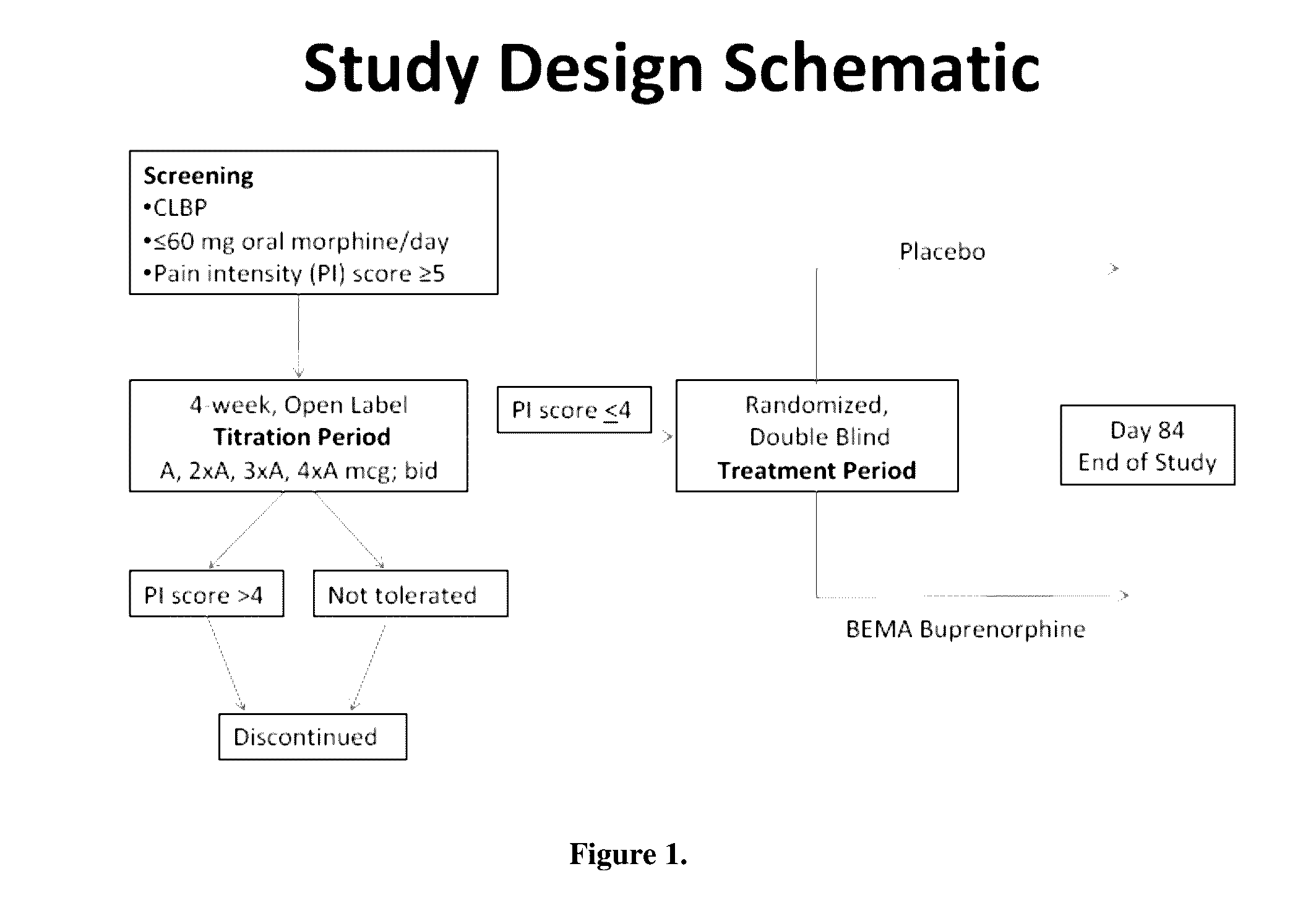
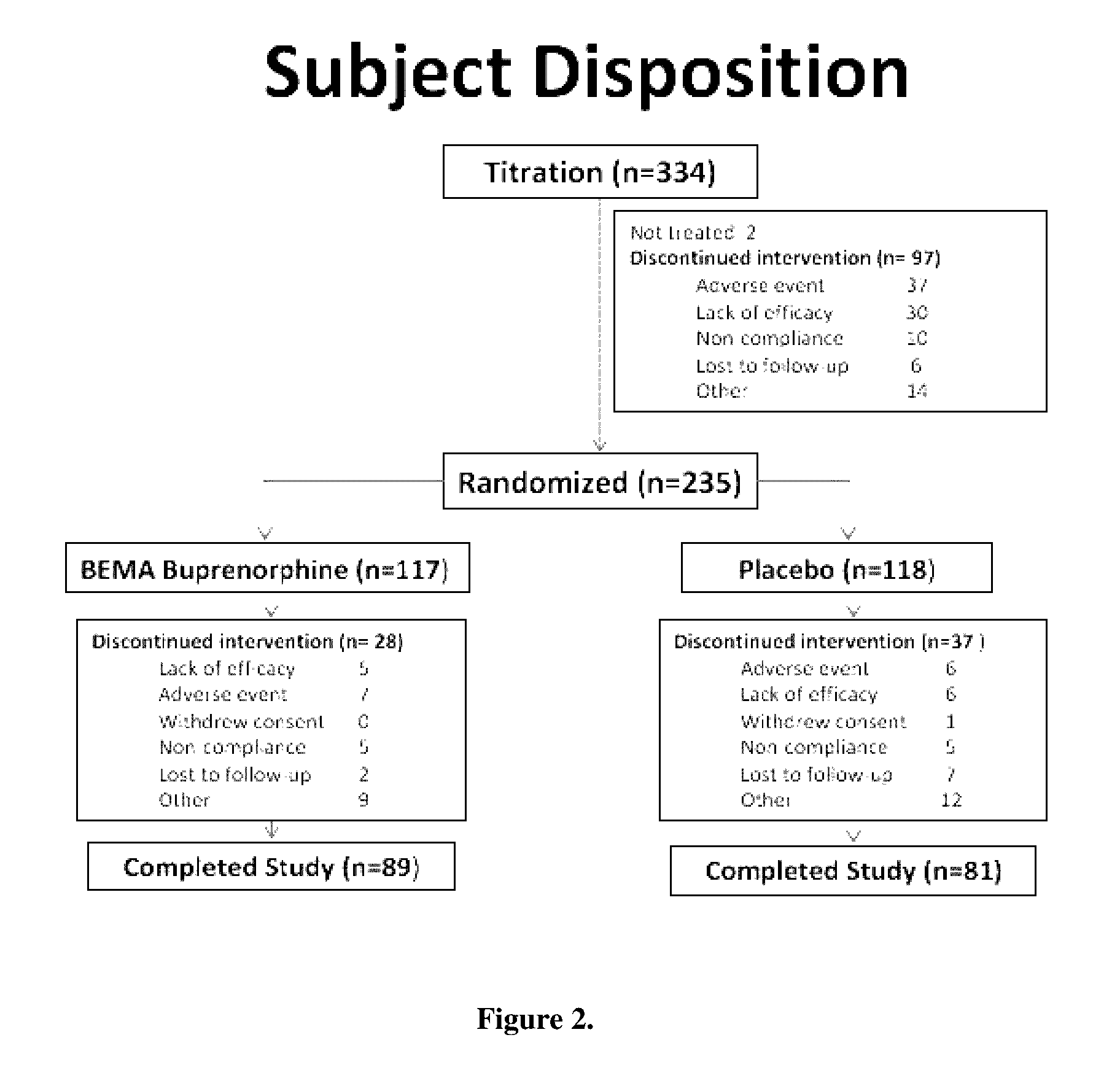
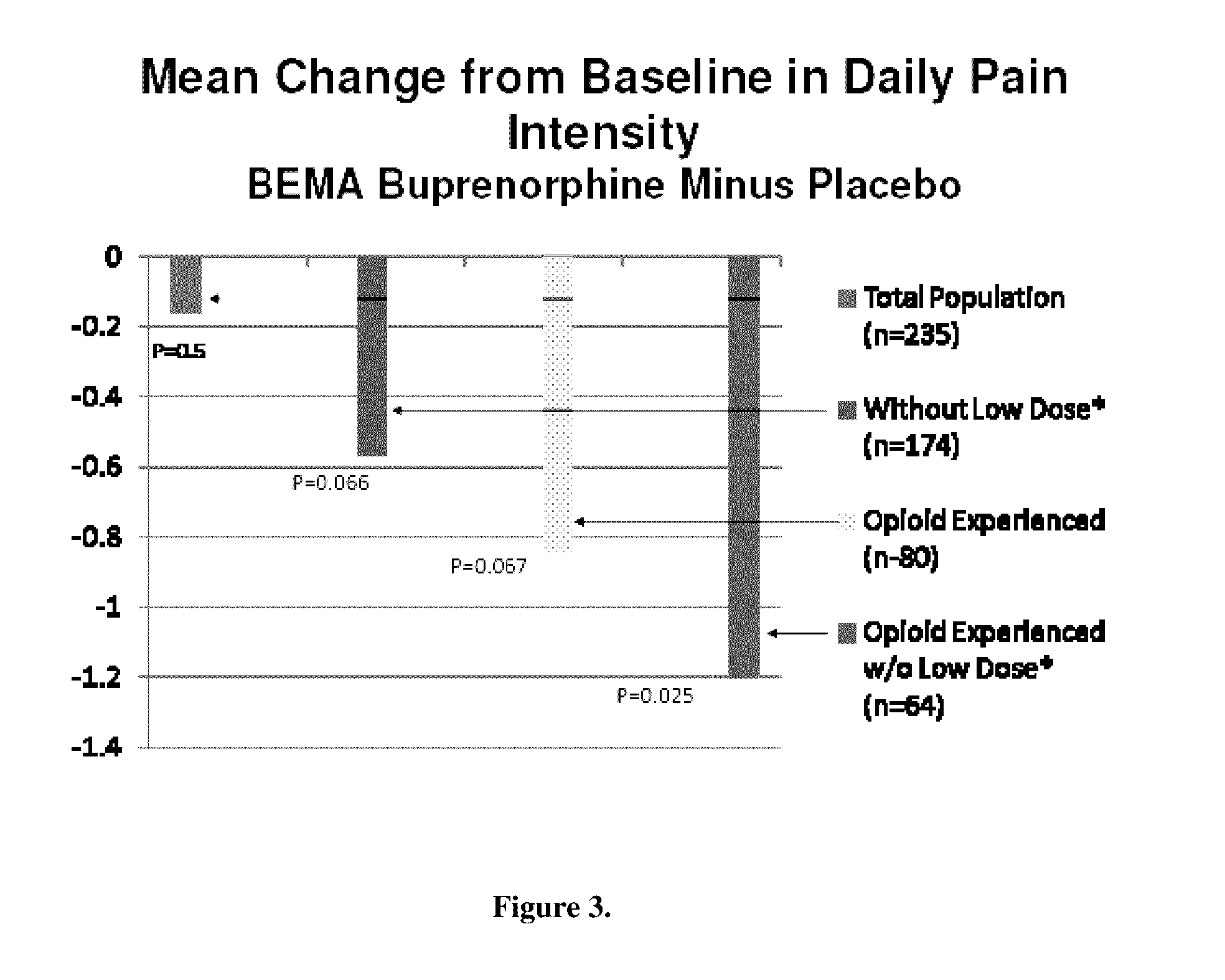
[0068]A 12-week, placebo-controlled, double-blind randomized withdrawal study was conducted to evaluate the efficacy and safety of buprenorphine delivered twice daily via transmucosal drug delivery device with enhanced uptake (BEMA buprenorphine) in subjects with moderate to severe chronic low back pain. The study was also designed to define the range of BEMA Buprenorphine doses effective for management of moderate to severe chronic lower back pain.
i. Study Design
[0069]The study consisted of an open-label dose titration period lasting up to 4 weeks, followed by a randomized, double-blind, placebo-controlled treatment period of 12 weeks. The subjects continued on their current pain therapy during the initial screening period (days −14 to −1) and until 12 to 24 hours prior to Day 0 / 1 of the open-label dose titration period. Predose assessments ...
example 3
Pharmacokinetic Profiles for BEMA Buprenorphine
[0083]Pharmacokinetic parameters for the BEMA Buprenorphine doses used in the treatment of chronic pain were determined in a separate, multiple dose study. BEMA Buprenorphine contained buprenorphine doses of 2×A μg, and 4×A μg. Each dose was administered twice daily for 3 days with serial blood samples collected. Selected pharmacokinetic parameters are shown in the Table 16 below.
TABLE 16Selected Pharmacokinetic Parameters for BEMA BuprenorphineBuccal Films comprising 1xA μg, 2xA μg, 3xA μg and4xA μg buprenorphinePharmacokinetic Parameters(Mean values)1xA μg2xA μg3xA μg4xA μgTmax (hr)2.902.612.002.20Cmax (ng / mL)0.07660.1560.2160.364Cmin (ng / mL)0.01570.03710.05580.0862Cavg (ng / mL)0.04090.08050.1130.195AUC0-τ (hr*ng / mL)0.49030.96581.3582.343AUClast (hr*ng / mL)0.40850.79021.1115.033Tmax refers to the time to reach the steady-state Cmax of plasma buprenorphine concentration.Cmax refers to the maximum concentration in plasma in steady-state.C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com