Cgrp receptor antagonist compounds for topical treatment of skin disorders
a technology of cgrp receptor and skin disorders, which is applied in the field of cgrp receptor antagonist compounds for topical treatment of skin disorders, can solve the problems of difficult diagnosis, limited response of skin immune system to internal and external stimuli, and discontinuation of skin inflammation development, so as to reduce the effect of toxic side effects and reduce the effect of skin inflammation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
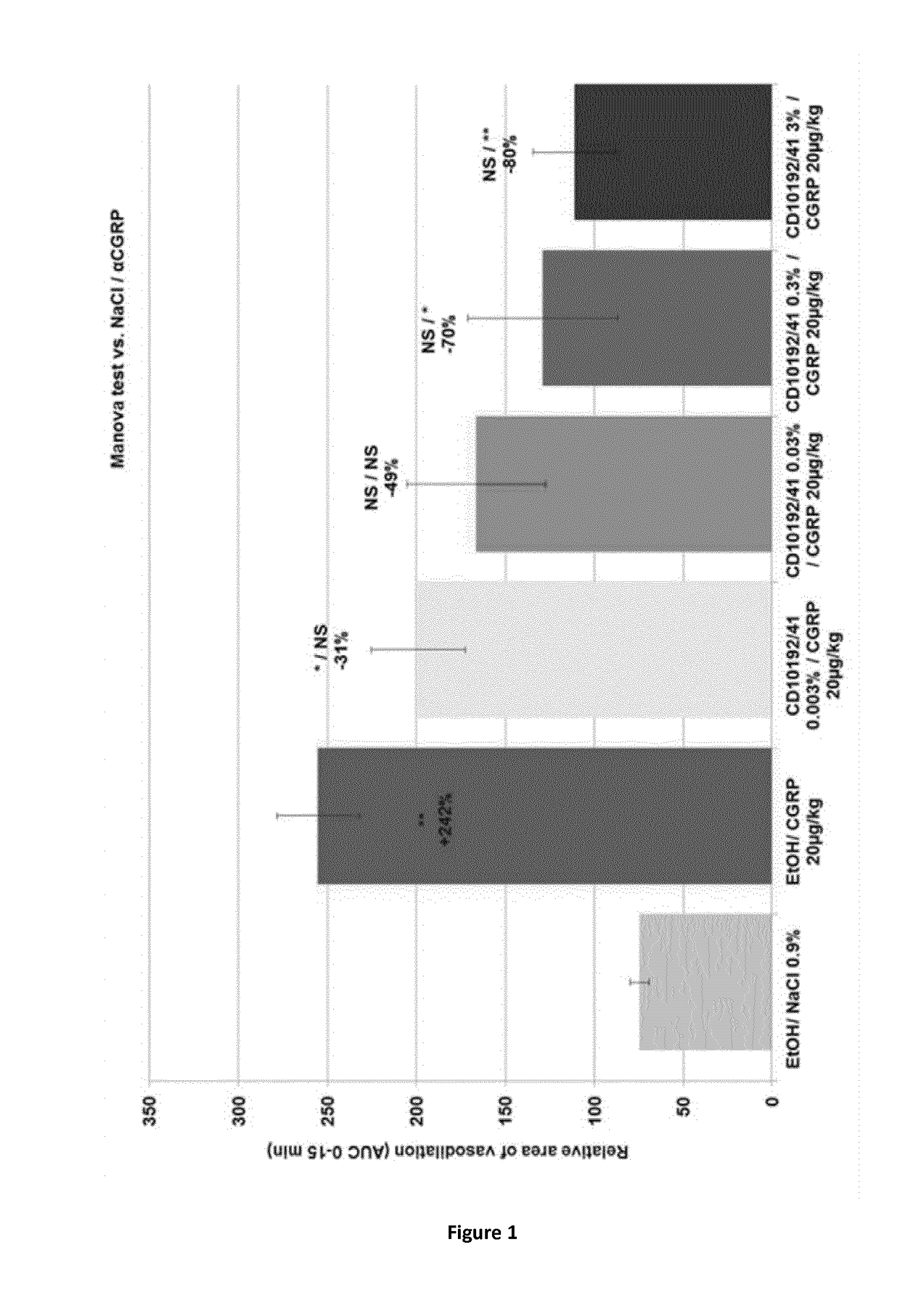
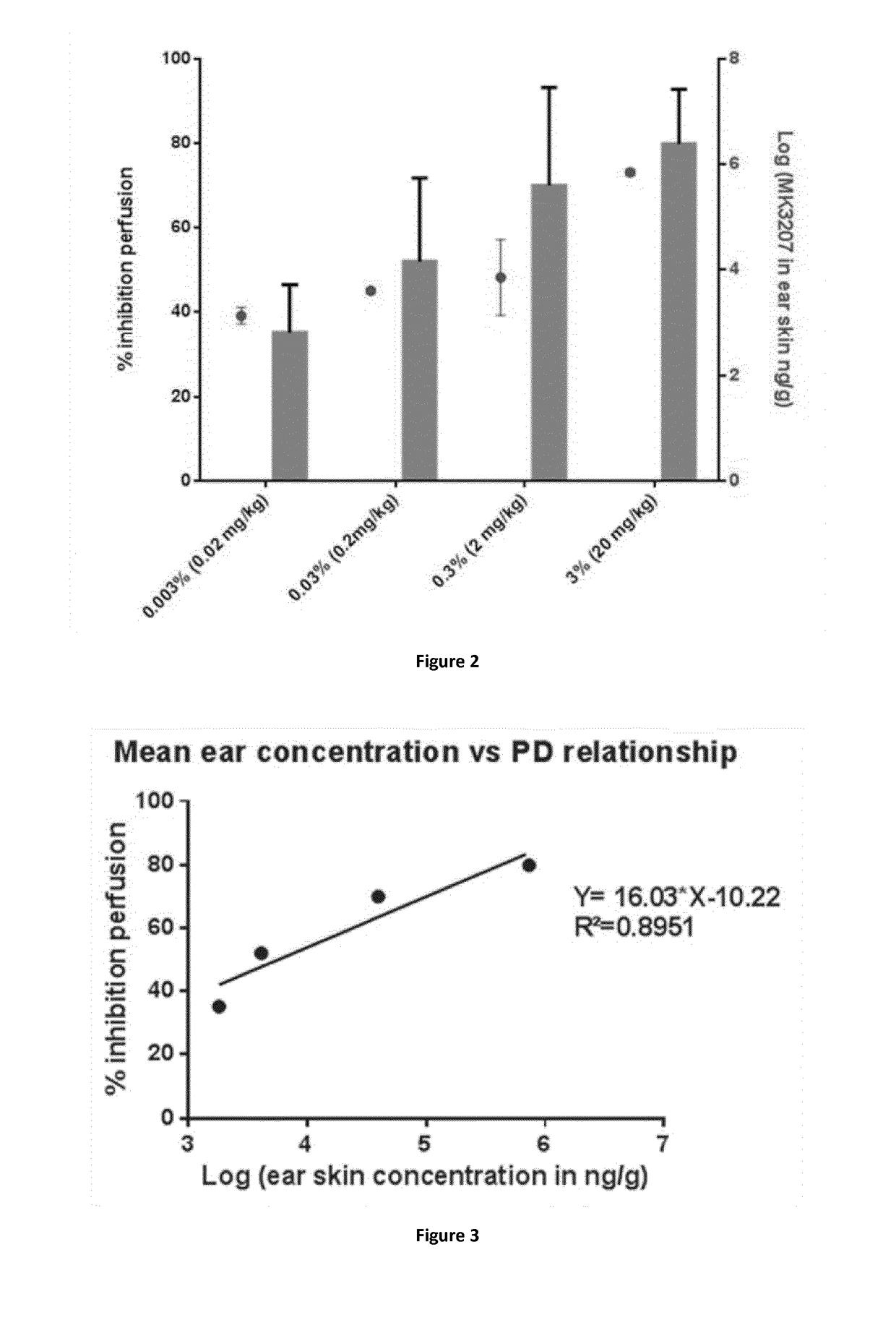
Evaluation of the Pharmacodynamic Activity of MK3207 (Herein Also Designated CD10192 / 41) Following Topical Treatment in a CGRP-Induced Mice Model of Vasodilatation
Materials and Methods:
[0413]This study has been conducted on BALB / c mice, 5 mice per group have been used. Animals were topically treated with multiple doses of MK3207 (0.003% to 3%) in ethanol or with ethanol alone (20 μl, right ear), 90 minutes before the intravenous injection of αCGRP 20 μg / kg (2 hours before sampling).
[0414]Anaesthesia was performed 15 minutes before the intravenous injection of αCGRP or NaCl 0.9%. Body temperature was maintained at 35° C.-37° C. using an automated heating pad. Images were obtained from Laser Doppler perfusion imaging PIM3 (Perimed, France). For each mouse, the skin blood perfusion was evaluated every minute on the right ear. The laser beam of the PIM3 scanned a skin area of 2×2 cm. Prior to the treatment, 2 scans were performed.
[0415]The duration of the recording was 22 minutes in tot...
example 2
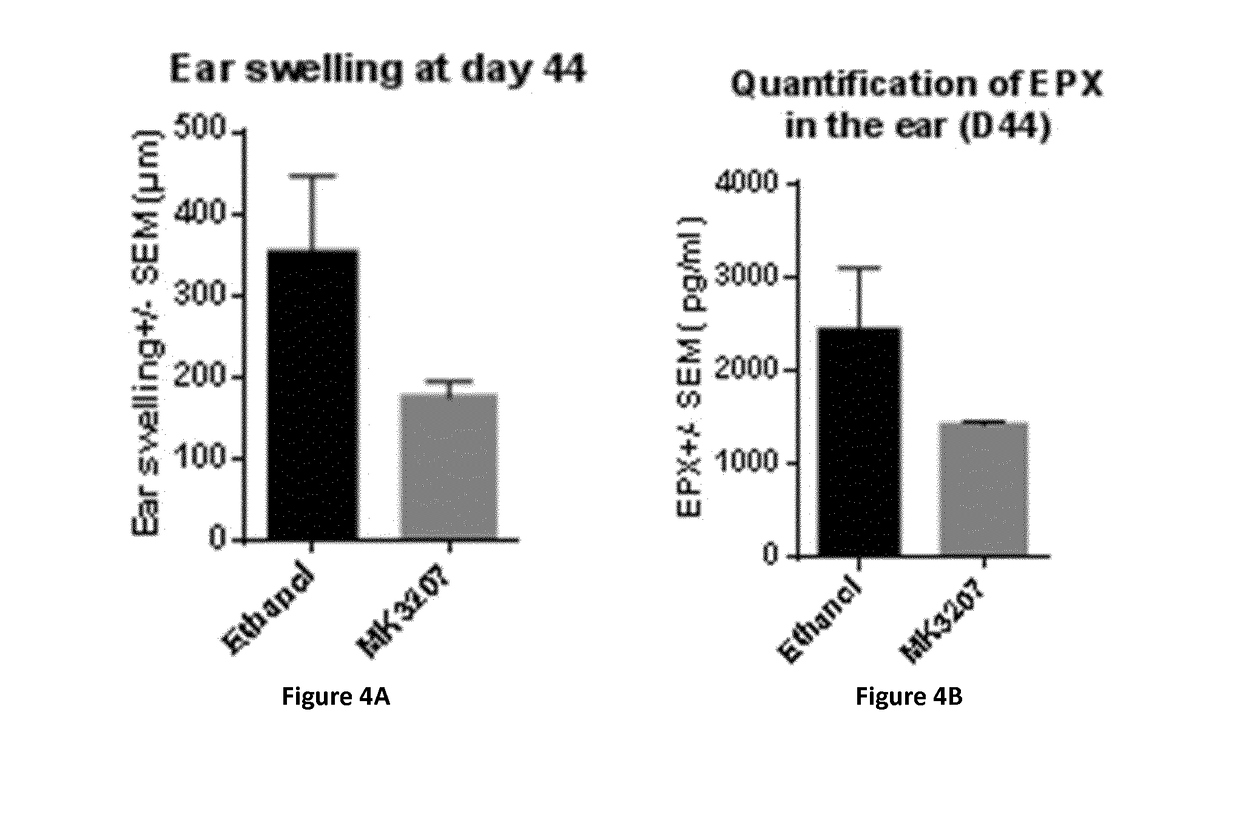
Evaluation of the Anti-Inflammatory Effect of MK3207 (Herein Also Designated CD10192 / 41) in an Experimental Model of Atopic Dermatitis in Mouse
[0425]1. Material and methods:[0426]1.1. Der F-Induced AD Mouse Model
[0427]The Dermatophagoides farinae (Der f)-induced AD mouse model has been shown to be suitable for the study of the atopic dermatitis pathophysiology and the evaluation of new therapies (J. Invest. Dermatol., 2009, 129, 31-40). Der f has been purchased from Greer Laboratories and a Der f solution has been prepared in 70% DMSO in MilliQ water. 375 μg of Der f has been applied during 7 weeks on the left ear of mice. Compounds to be tested were applied every day of the week except during the weekend, on the Der f treated ear from week 4 until day 44.[0428]1.2. Compounds[0429]1.2.1. MK3207 (CD10192 / 41)
[0430]MK3207 (CD10192 / 41) has been provided by Galderma and 3% of MK3207 (CD10192 / 41) solutions in ethanol have been prepared.[0431]1.2.2. Triamcinolone Acetonide
[0432]Triamcinolo...
example 3
Evaluation of the Effect of MK3207 (Herein Also Designated CD10192 / 41) in an Experimental Model of Psoriasiform Mice
[0445]1. Material and Methods[0446]1.1 Imiquimod Induced Psoriasiform mice Model
[0447]Imiquimod psoriasiform model has been induced by daily topical application of Aldara (3.18 mg of imiquimod) for 3 or 7 days on the shaved back skin of Balb / c mice. The pharmacodynamic analysis were performed by using a transcriptional analysis at D4, a histological analysis at D4 and D8, a cells analysis at D8 and a Cytokines analysis at D8.[0448]1.2. Compounds[0449]1.2.1. Imiquimod
[0450]Imiquimod is sold under the name Aldara™ 5% by 3M-Pharmaceutical. 63.5 mg of Aldara cream is applied on the back skin of the mice, representing 3.2 mg of active compound.[0451]1.2.2. MK3207 (CD10192 / 41)
[0452]MK3207 compound is dissolved in PG / Ethanol 96 (30 / 70) and applied twice daily 2 h 00 before and 6 h 00 after Aldara treatment.[0453]1.2.3. Vehicles
[0454]PG / Ethanol (30 / 70) is used as a vehicle.[04...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ea | aaaaa | aaaaa |
| Ra | aaaaa | aaaaa |
| enantiomers | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com