Anti-death receptor antibodies and methods of use thereof
a technology of anti-death receptor and antibody, which is applied in the field of monospecific or bispecific antibodies, can solve the problems of limited clinical efficacy and achieve the effects of facilitating antibody clustering, enhancing potency, and enhancing potency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
s and Antigens
[0362]Expression constructs for antibodies For antibody expression variable heavy (VH) chain and Variable light (VL) chain sequences were cloned in pcDNA3.3 expression vectors containing IgG1 heavy chain (HC) and light chain (LC) constant regions. Desired mutations were introduced either by gene synthesis or site directed mutagenesis. Antibodies mentioned in this application have VH and VL sequences derived from previously described chimeric human / mouse DR5 antibodies DR5-01 and DR5-05 (based on EP2684896A1), humanized DR5 antibodies hDR5-01 and hDR5-05 (based on WO2014 / 009358), IgG1-CONA (based on U.S. Pat. No. 7,521,048 B2 and WO2010 / 138725), IgG1-chTRA8 (based on EP1506285B1 and U.S. Pat. No. 7,244,429B2), IgG1-DR5-H48-2 (based on US 2004 0214235 A1), IgG1-DR4-T1014G03 (based on U.S. Pat. No. 7,361,341), and IgG1-FAS-E09 (based on Chodorge et al., Cell Death Differ. 2012 July; 19(7): 1187-1195). In some of the examples the human IgG1 antibody b12, a gp120-specific a...
example 2
ion of a Hexamerization-Enhancing Mutation does not Affect Binding of IgG1-DR5-01-K409R, IgG1-DR5-05-F405L and Bispecific Antibody IgG1-DR5-01-K409R×DR5-05-F405L to DR5-Positive Human Colon Cancer Cells
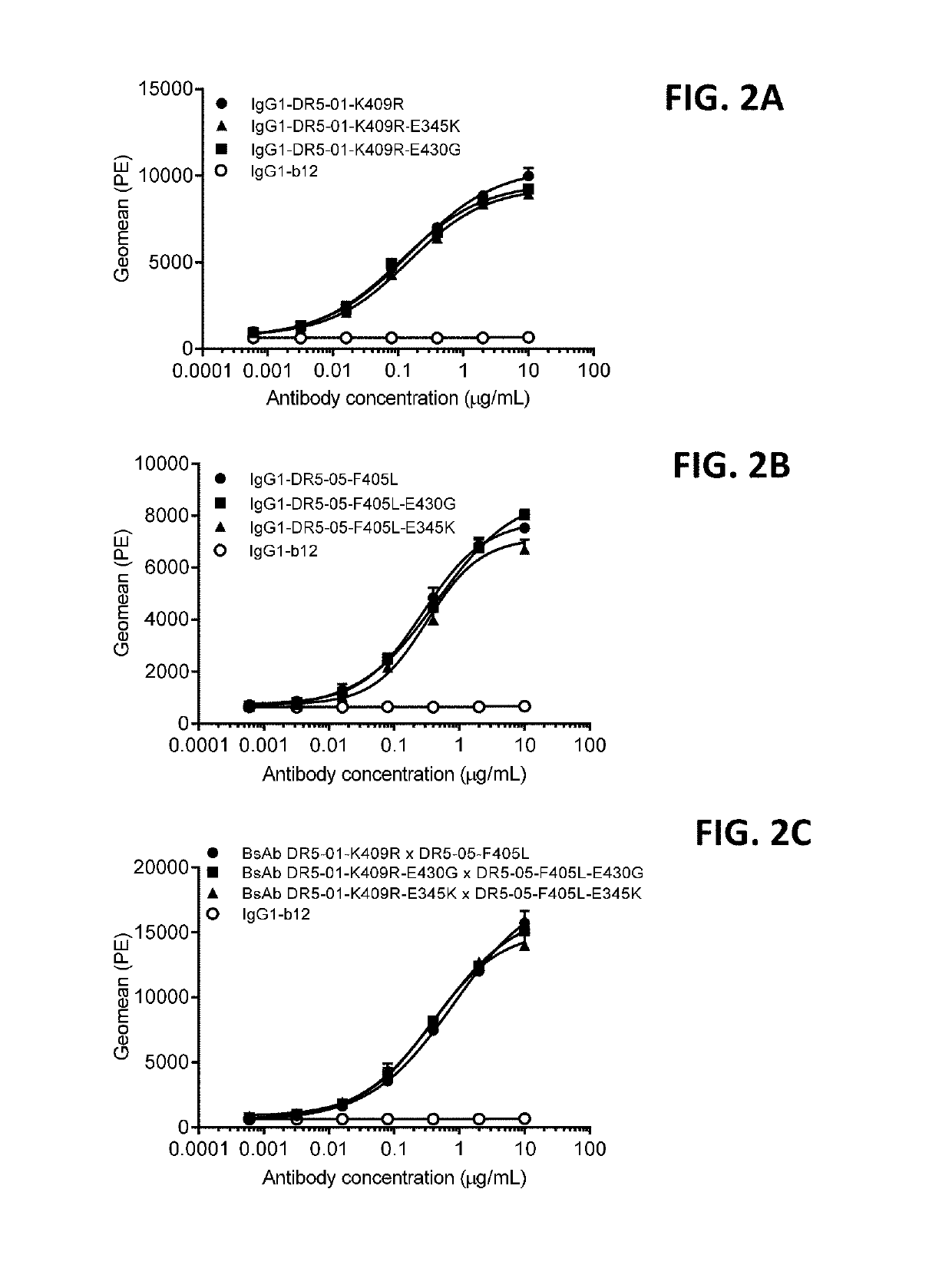
[0366]Binding of purified antibody variants of IgG1-DR5-01-K409R, IgG1-DR5-05-F405L and bispecific antibody IgG1-DR5-01-K409R×IgG1-DR5-05-F405L (BsAb DR5-01-K409R×DR5-05-F405L) with and without a hexamerization-enhancing mutation (E430G or E345K) to human colon cancer cells COLO 205 was analyzed by FACS analysis. Cells were harvested by pooling the culture supernatant containing non-adherent cells and trypsinized adherent COLO 205 cells. Cells were centrifuged for 5 minutes at 1,200 rpm and resuspended in 10 mL culture medium [RPMI 1640 with 25 mM Hepes and L-Glutamine (Lonza Cat nr BE12-115F)+10% Donor Bovine Serum with Iron (Life Technologies Cat nr 10371-029)+50 Units Penicillin / 50 Units Streptomycin (Lonza Cat nr DE17-603E)]. Cells were counted, centrifuged again and resuspended i...
example 3
ion of a Hexamerization-Enhancing Mutation does not Affect Binding of DR4 Antibody to Soluble Human DR4
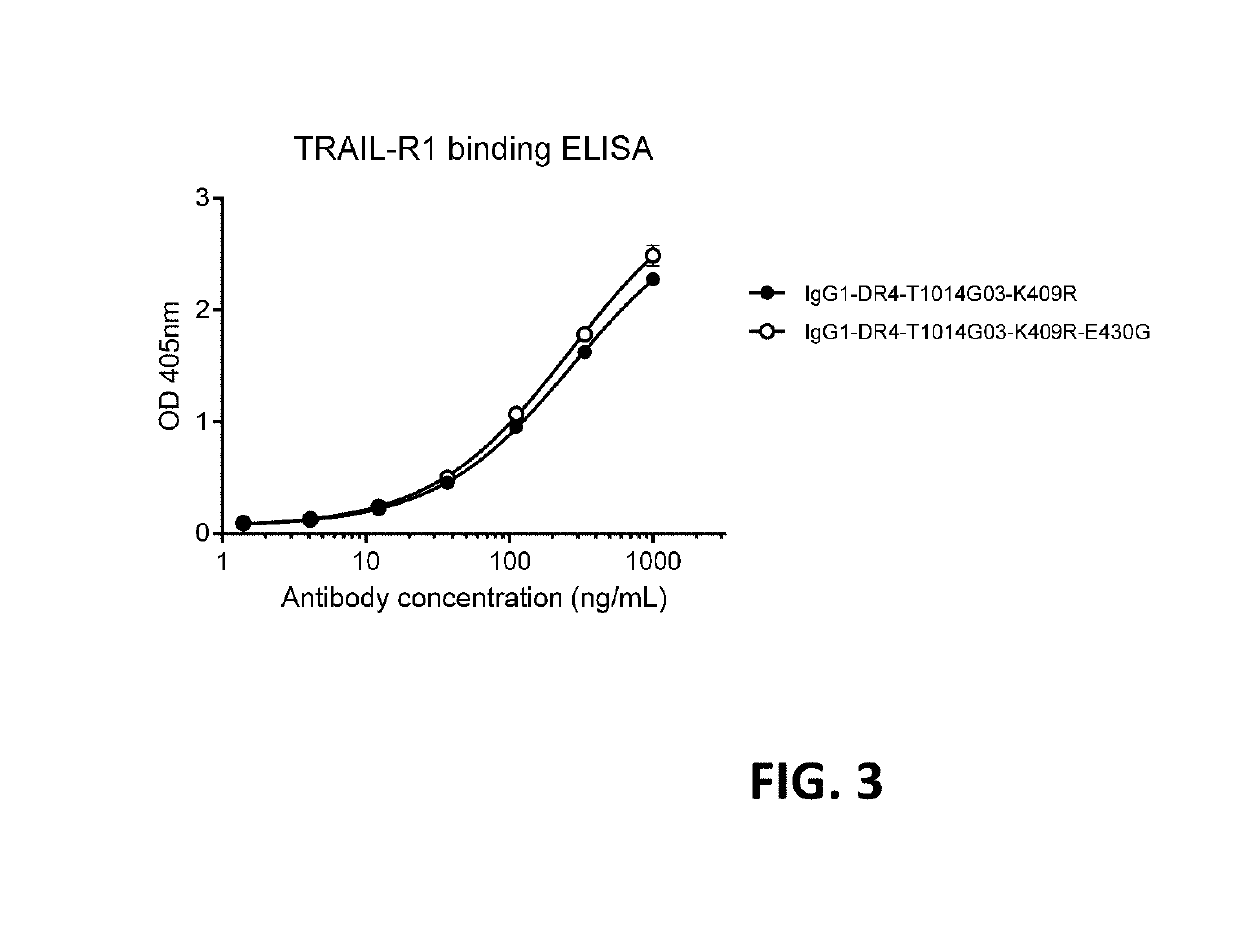
[0368]Binding of purified antibody variants of IgG1-DR4-T1014G03 with and without hexamerization-enhancing mutation E430G to coated human soluble DR4 was analyzed in a sandwich enzyme-linked immunosorbent assay (ELISA). 96-well flat bottom ELISA plates (Greiner bio-one; Cat nr 655092) were coated overnight at 4° C. with 2 μg / mL sTRAIL-R1 (Peprotech cat nr 310-18) in 100 μL PBS. The wells were washed three times with PBST [PBS with 0.05% Tween-20 (Sigma-Aldrich; Cat nr 63158)]. The wells were blocked by adding 200 μL PBSA [PBS with 1% Bovine Serum Albumin (BSA; Roche Cat #10735086001)] and incubated for 1 hour at room temperature while shaking. The wells were washed three times with PBST. Next, antibody samples of IgG1-DR4-T1014G03-K409R or IgG1-DR4-T1014G03-K409R-E430G (range 0 to 2,000 ng / mL final concentrations in 3-fold dilutions) were added in a total volume of 100 μL PBSTA (PB...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com