Hyaluronic Acid Binding Domain-Growth Factor Fusion Protein cDNAs and Fusion Proteins for Cartilage Matrix Preservation and Report
a technology of hyaluronic acid and growth factor, which is applied in the direction of fusions for specific cell targeting, hormone peptides, genetic material ingredients, etc., can solve the problems of limited factors, unsolved clinical problems for articular cartilage repair, and damage to articular cartilage, so as to increase the responsiveness rate of chondrocytes, and reduce gf removal rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
and Methods
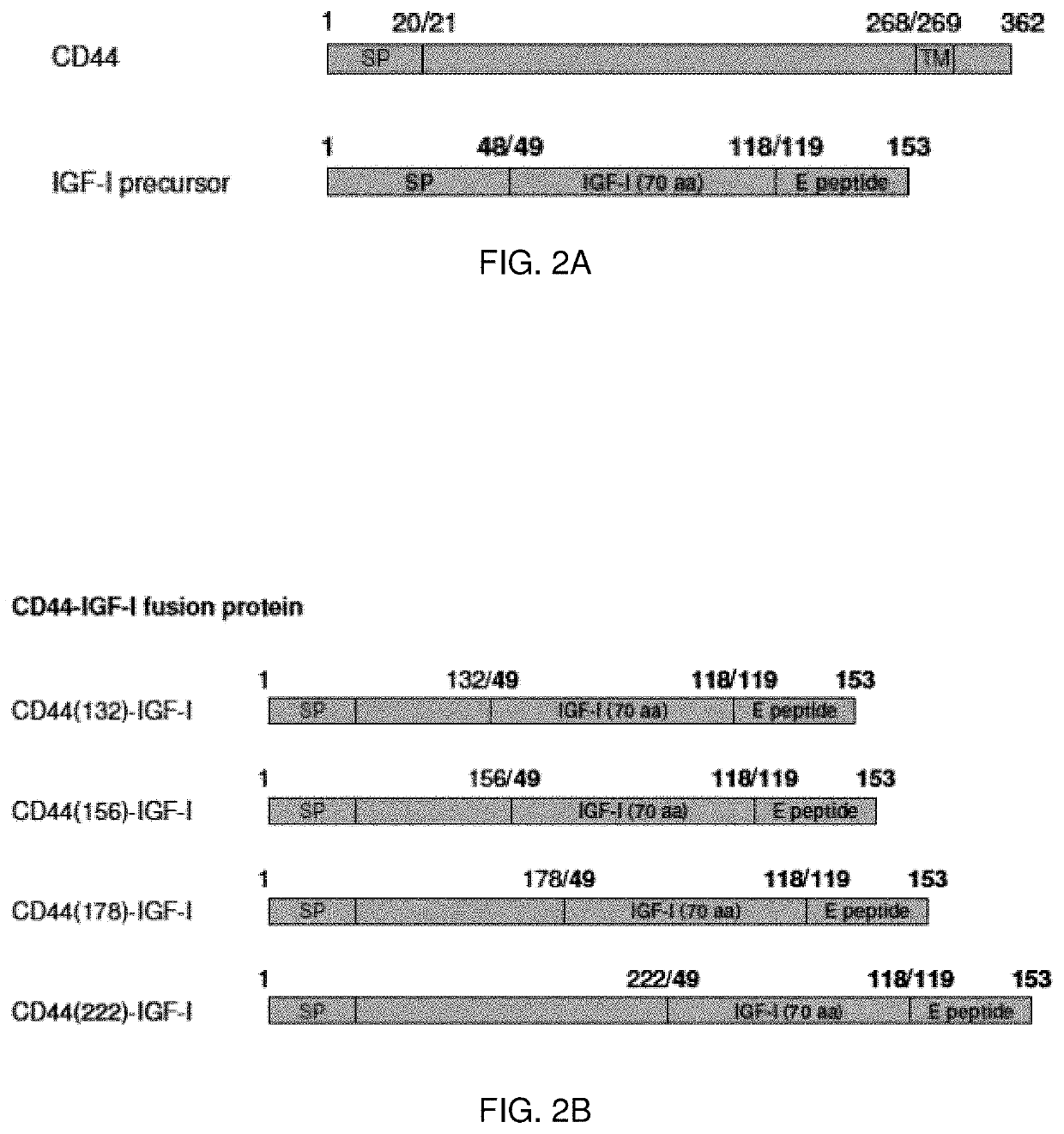
[0124]Fusion Proteins
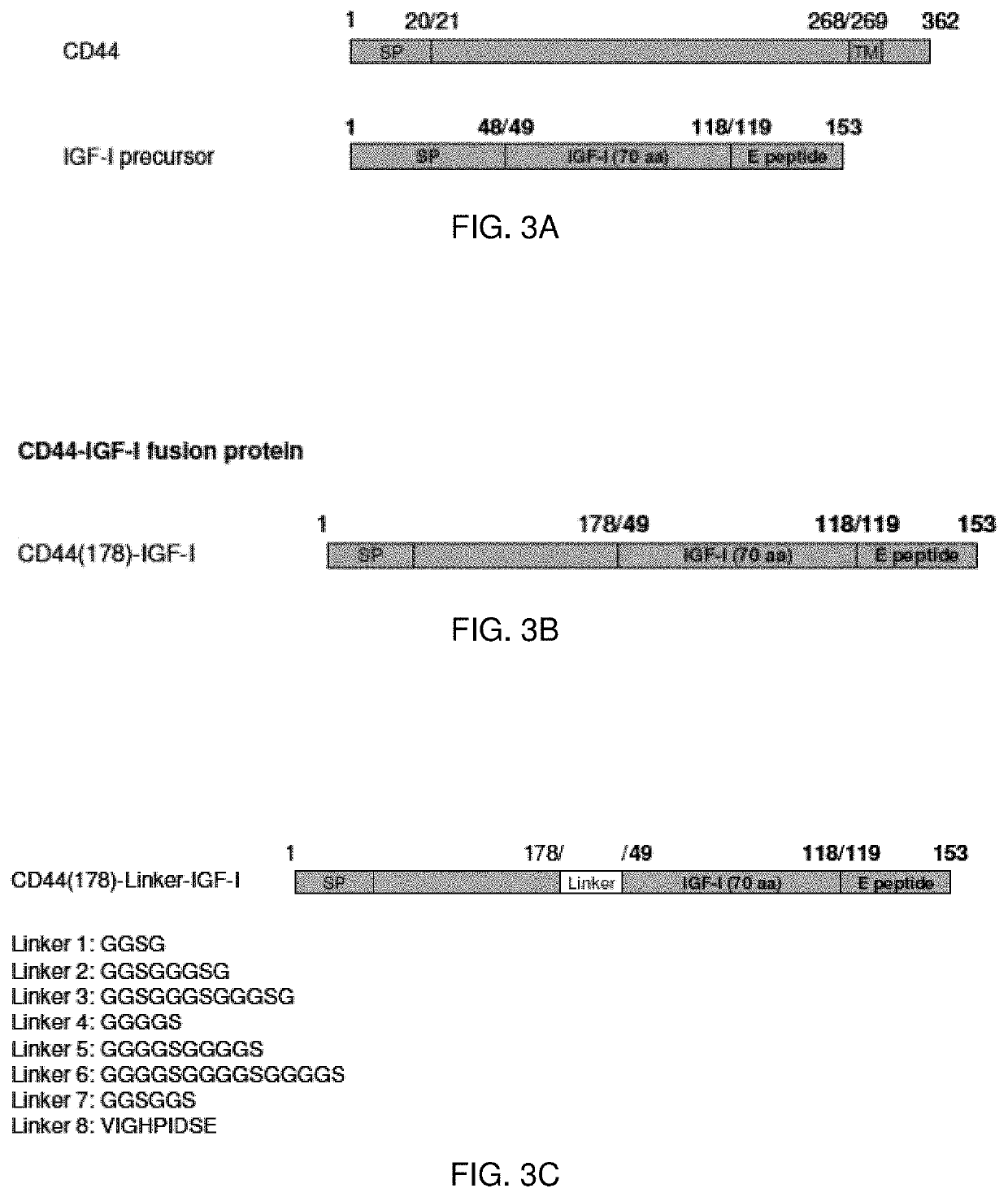
[0125]The nucleic acid sequences encoding the described proteins and chimeric nucleic acid sequences encoding fusion proteins may be constructed. The chimeric nucleic acid molecules may be prepared with sections encoding a linking peptide connecting the protein portions of the encoded fusion protein. Optionally, the peptide linker may be selected to include residues imparting stearic flexibility in order to enhance the function of the fusion protein.
[0126]Fusion Protein Linkers
[0127]The components of the fusion proteins may be operatively linked directly one to the other. The two protein components of the fusion protein may be directly linked via an amino terminus to carboxy terminus peptide bond.
[0128]Alternatively, one or more linker molecules connect the two portions of the fusion protein. The linker molecule allows the two portions of the fusion protein increased stearic freedom. In one embodiment, the linkers are peptide linkers. Peptides for lin...
example 2
of Hyaluronic Acid Binding—Insulin-Like Growth Factor I Fusion Protein cDNA Constructs
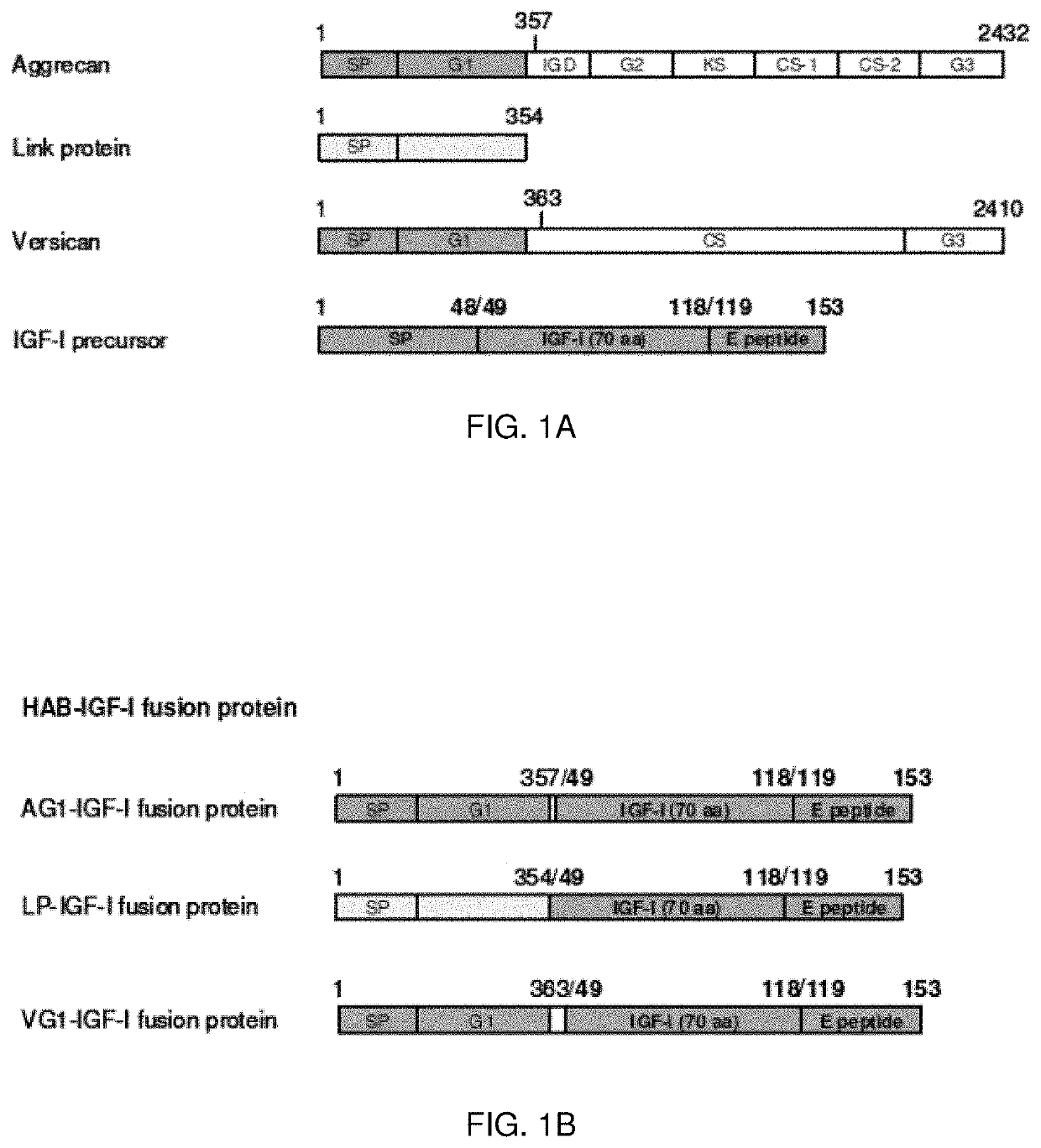
[0158]Three cDNAs were created, encoding three hyaluronic acid-binding domain (HAB)—insulin-like growth factor I (IGF-I) fusion proteins (HAB-IGF-I) by coupling the sequence encoding IGF-I with those encoding a hyaluronic acid binding region derived from three cartilage matrix proteins. These regions include 1) the aggrecan G1 domain (AG1), 2) the versican G1 domain (VG1) and 3) link protein (LP). The resulting fusion proteins are designated AG1-IGF-I, VG1-IGF-I, and LP-IGF-I respectively (FIG. 1B). Further, the encoded fusion proteins were created by transferring the cDNAs into producer cells and the selected therapeutic target cells, articular chondrocytes.
[0159]To facilitate the HAB-IGF-I constructs to be used for human therapeutic purposes, the DNA constructs encoding all the HAB-IGF-I fusion proteins were generated from native human matrix protein gene sequences and the native human IGF-I gene...
example 3
n of Fusion Proteins and Demonstration of Fusion Protein Integrity
[0162]To promote articular cartilage preservation or repair using these fusion proteins as therapeutic agents, it is necessary to have a production source for the proteins. A method of production employs the fusion protein-encoding cDNAs described above and a non-viral (plasmid) vector that the inventors developed previously, Shi et al., Effect of Transfection Strategy on Growth Factor Overexpression by Articular Chondrocytes, J. of Orthopaedic Research, January 2010, hereby incorporated by reference. The cDNAs were inserted into the vector and used to transfect HEK 293 cells (Stratagene). The HEK 293 cells then use these inserted transgenes to synthesize the HAB-IGF-I fusion proteins. On day 3 after transfection conditioned medium was collected for measurement of HAB-IGF-I fusion proteins produced as transgene products by the HEK 293 cells. The production and integrity of these proteins was confirmed by IGF-I ELISA (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volumes | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com