Device for treatment of valve regurgitation
a technology for valve regurgitation and implantable devices, which is applied in the field of implantable devices for treating heart valve regurgitation, can solve the problems of serious health diseases such as regurgitation, heart failure and death of patients, and patients' heart failure and death, and achieve the effect of effective treatment methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment 1
[0051]A device for treating mitral regurgitation, comprises a frame and an anchoring device with the anchoring device being connected to the frame.
[0052]Frame
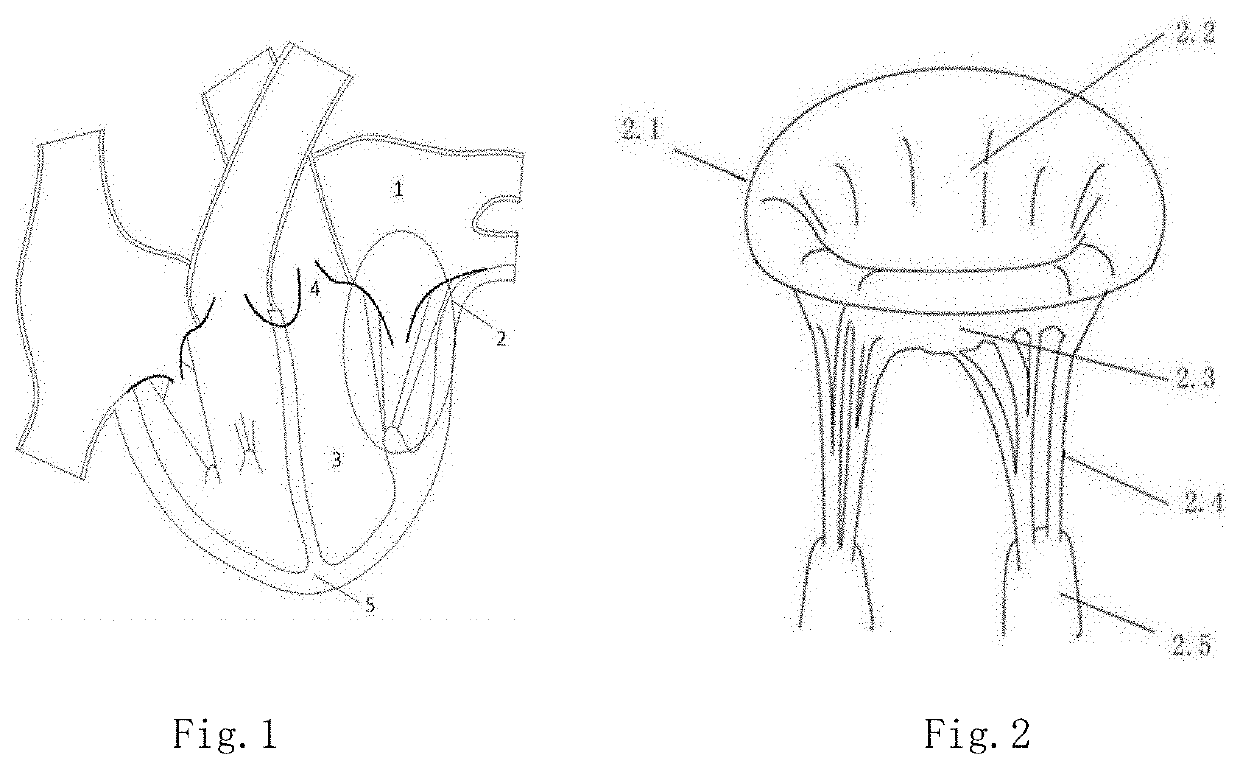
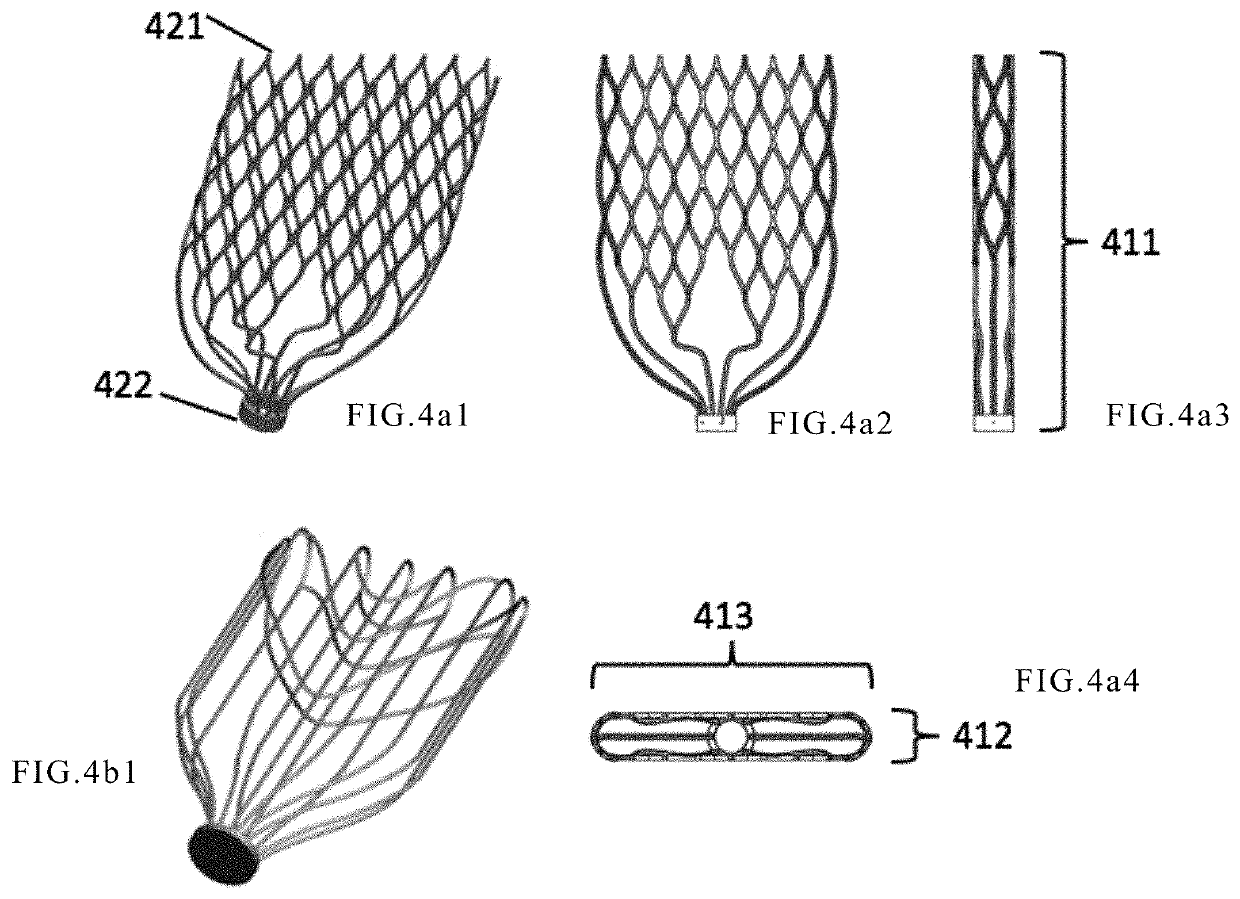
[0053]The frame is placed in the native mitral valve leakage gap by an anchoring device when the mitral valve is closed, the frame being expanded at the gap, the native valve leaflets being in close contact with the outer surface of the frame when it is closed. The frame has an inflow end and an opposite outflow end. The frame acts as a supporting structure for a prosthetic valve in the frame and makes the entire device compressible and expandable.
[0054]In vitro, the device is compressed into the catheter, and upon reaching a predetermined position of the native valve orifice, the device is pushed out to self-expand back to its originally designed expanded state and is anchored by the anchoring device.
[0055]The outflow end of the frame is preferably tapered relative to the inflow end, which occupies less space and facilitates t...
embodiment 2
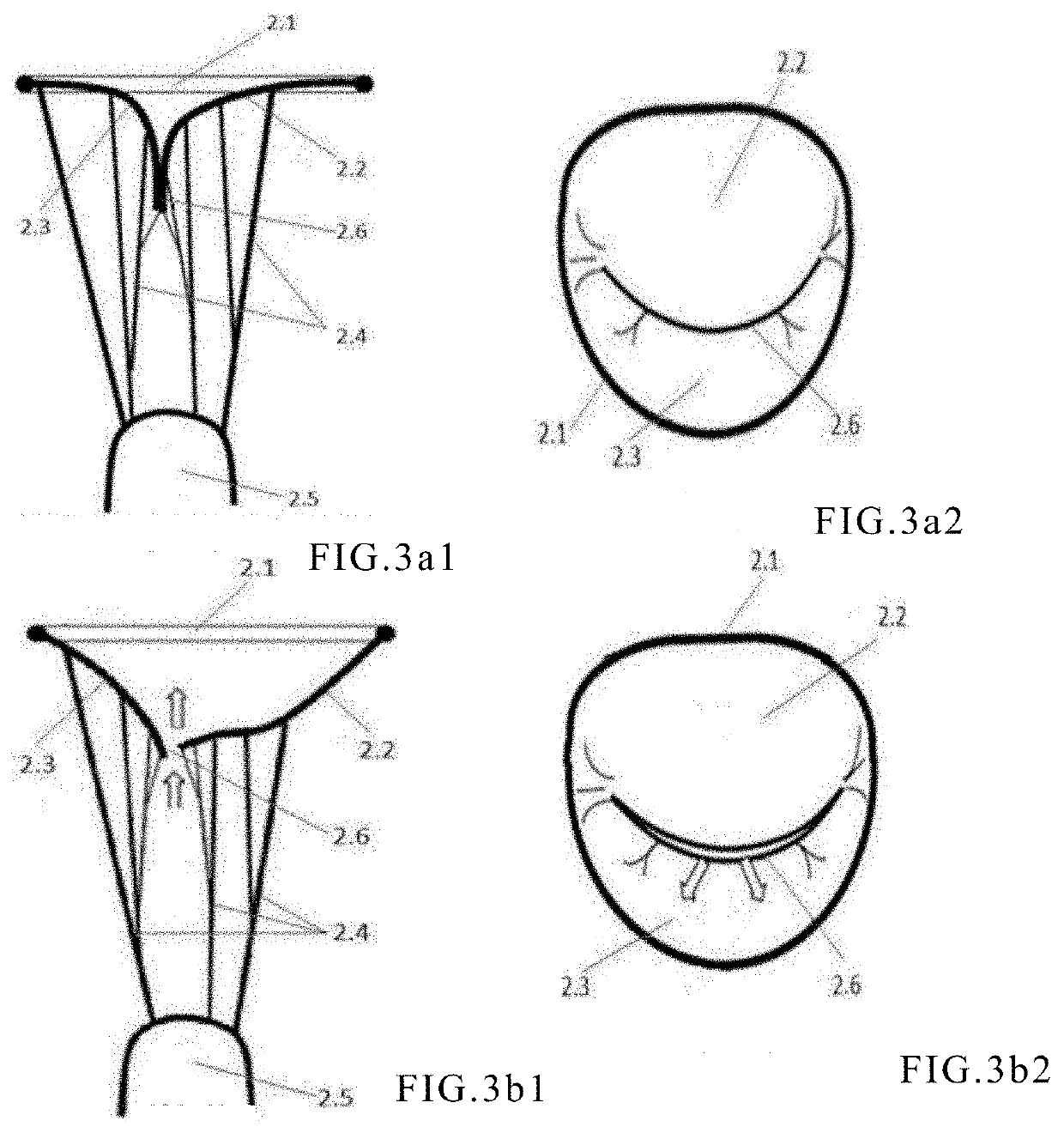
[0091]As shown by 101 to 106 in FIG. 10, the shape of cross-section in the short axis plane of the frame is an irregular combination of arcuate edges and may continuously vary from the inflow end to the outflow end of the frame, so as to fit different gaps between native valve leaflets during systole.
[0092]As shown in FIG. 10, is the cross-section of the frame in relationship to the native valve leaflets, in which 2.2 is the anterior leaflet and 2.3 is the posterior leaflet. In this case, the distribution of the gap 116 in the mitral regurgitation is not uniform; The gap is narrow in the middle, while the gaps at both sides may be irregular and may become rather large. Different from Embodiment 1, the shape 105 of the preferred cross-section of the embodiment of the present embodiment assumes a crescent-like shape. Its central portion has an arc with a height narrower than its two ends, and its two ends are rounder and larger. When the frame is in contact with the native valve leafl...
embodiment 3
[0093]Different from Embodiment 1, the anchor further includes an anchor mechanism involved with a lattice structure arranged inside the atrium, similar to the shape of a cage. The shape of the anchor can be adapted to the anatomy of the left atrium; so to allow positioning the frame at the desired location. The frame may be a lattice structure in a cylinder. When the inner diameter of the cylinder is large enough, it may displace the native valve to become a valve replacement. The distal end of the anchoring cage is connected to the inflow end of the frame, and may be connected by an elastic metal wire, such as a Ni—Ti alloy wire. The anchoring device, with elements including the linker supporting rod and the anchor as shown in the Embodiment 1, and the like, may be placed in the ventricle at the same time. As shown in FIG. 11, the anchoring device includes an intra-atrial lattice-like anchoring cage 132, a cylindrical lattice frame 133, and an intra-ventricular linker supporting r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com