Stable intranasal formulations of carbetocin
a technology of intranasal formulation and carbetocin, which is applied in the direction of drug composition, peptide/protein ingredient, metabolic disorder, etc., can solve the problems of increasing the likelihood of peptide aggregation, difficult to maintain a sufficient stability at high peptide concentration, and inability to optimize ph, etc., to achieve the effect of high concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
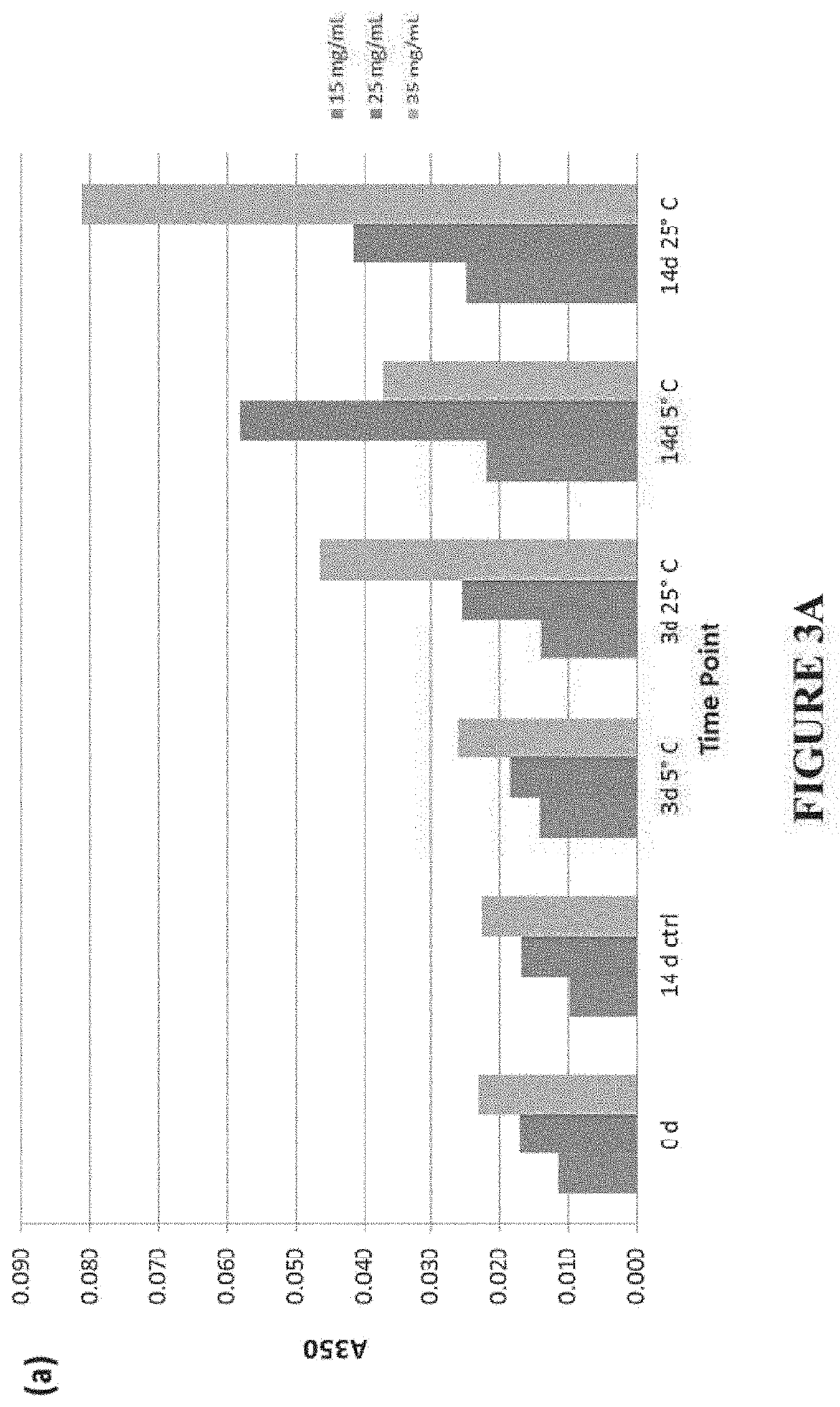
[0177]Carbetocin was obtained as a powder and was stored at ≤20° C. until ready for use. Formulations were prepared by dissolving 40 mg / mL or 20 mg / mL of carbetocin in an aqueous solution containing a solubilizer and / or HPMC. The pH of each formulation was adjusted to 5.4 and ±0.1 by addition of an appropriate amount of 5 M NaOH. All preparations were prepared using multi-compendial grade excipients and reagents, and ultra-pure water (Millipore MilliQ, 18 MΩ). The osmolality of each preparation was measured before preparing the final formulation to ensure it was similar to that of the theoretically determined value. Each formulation (bulk material) was sterile filtered using a Millipore Millex-GV syringe filter (0.22 μm). 1.2 mL of each sterile filtered formulation was filled into a 3 mL glass vial, stoppered with a 13 mm Fluorotec coated serum stopper, and crimped. All materials (i.e., vials, stoppers, etc.) were sterilized before filling. For samples with reduced or limited headsp...
example 2
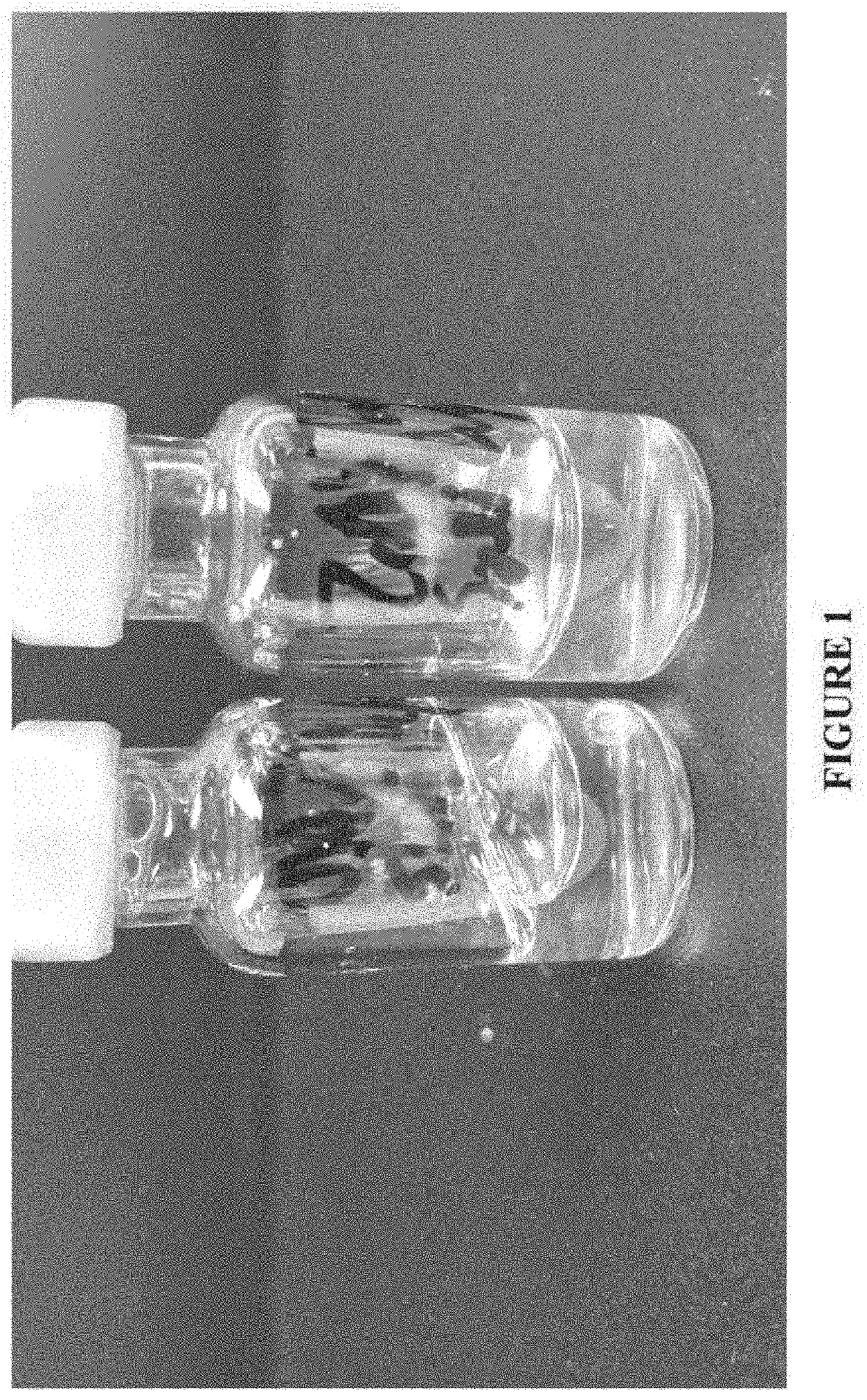
[0181]Samples were prepared using the general procedure provided in Example 1. It is noted that the hydrotropes studied in this example were formulated at the following concentrations: 160 mM (isotonic) and 400 mM sodium benzoate, 200 mM (isotonic) and 400 mM sodium salicylate, and 82 mM caffeine (near solubility limit), and 35 mg / mL carbetocin. Again, as in Example 1, an agitation study was conducted to evaluate the ability of these solutions to suppress particle formation upon agitation. Observations were made after both 14 and 24 hours of agitation.
[0182]After 14 hours, the following was observed: the benzoate preparations / samples (160 mM and 400 mM) formed a hard precipitate. The caffeine preparation formed a carbetocin skin on the vial wall. The salicylate preparations formed a few fine particles, but were otherwise generally clear. After 24 hrs of agitation, the 200 mM salicylate preparation had slightly more particles / precipitate than its 400 mM counterpart. Additionally, the...
example 3
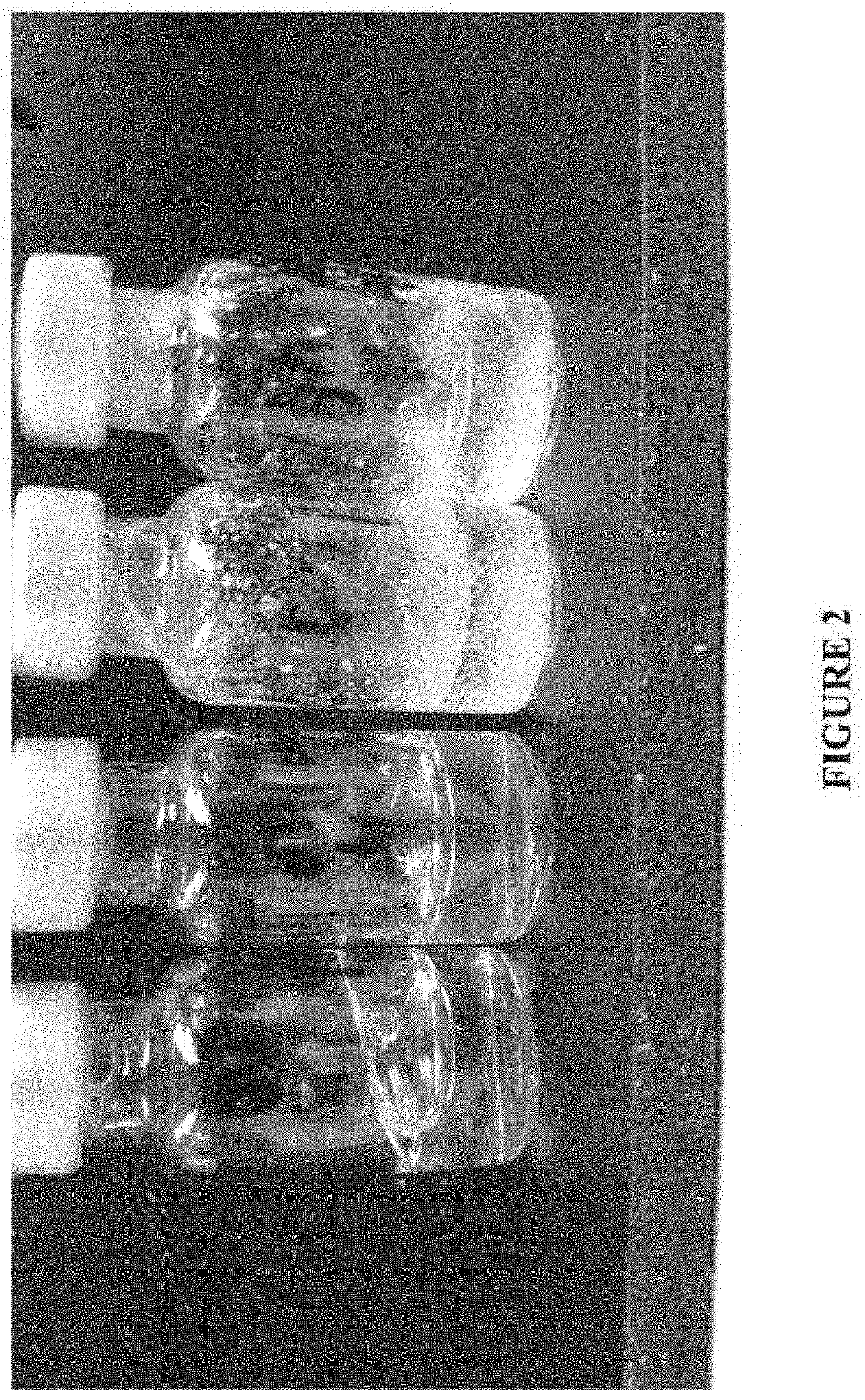
[0185]Formulations were prepared according to the method described in Example 1 by dissolving the desired amount of 40 mg / mL of carbetocin in an aqueous solution containing different excipients or HPMC. The pH of each formulation was adjusted to 5.4 and ±0.1 by addition of an appropriate amount of 5 M NaOH. After sterile preparation according to the same method described in Example 1, samples were placed horizontally on an orbital plate shaker (Labnet, 3 mm orbit) and shaken continuously at 200 rpm for prescribed periods of time. Samples were shielded from ambient light during agitation. All samples used in this study were agitated at room temperature. The results of this experiment are summarized below in Tables 2 and 3.
TABLE 2Visual Observation Results for Agitated Carbetocin FormulationsCarbetocinVialAgitation TimeConcentrationExcipientOrientation(hrs)Observations40 mg / mL DSHydroxypropyl β-CyclodextrinHorizontal17Significant precipitation40 mg / mL DS1% (w / v) Hydroxypropyl cellulos...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com