Compositions and Methods for Pesticide Degradation
a technology of pesticide degradation and composition, applied in the field of compositions and methods concerning pesticide degradation, can solve the problems of affecting the appearance and quality of crops and fruits sprayed with massive amounts of pesticide, affecting the health of people, so as to reduce the half-life of pesticides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
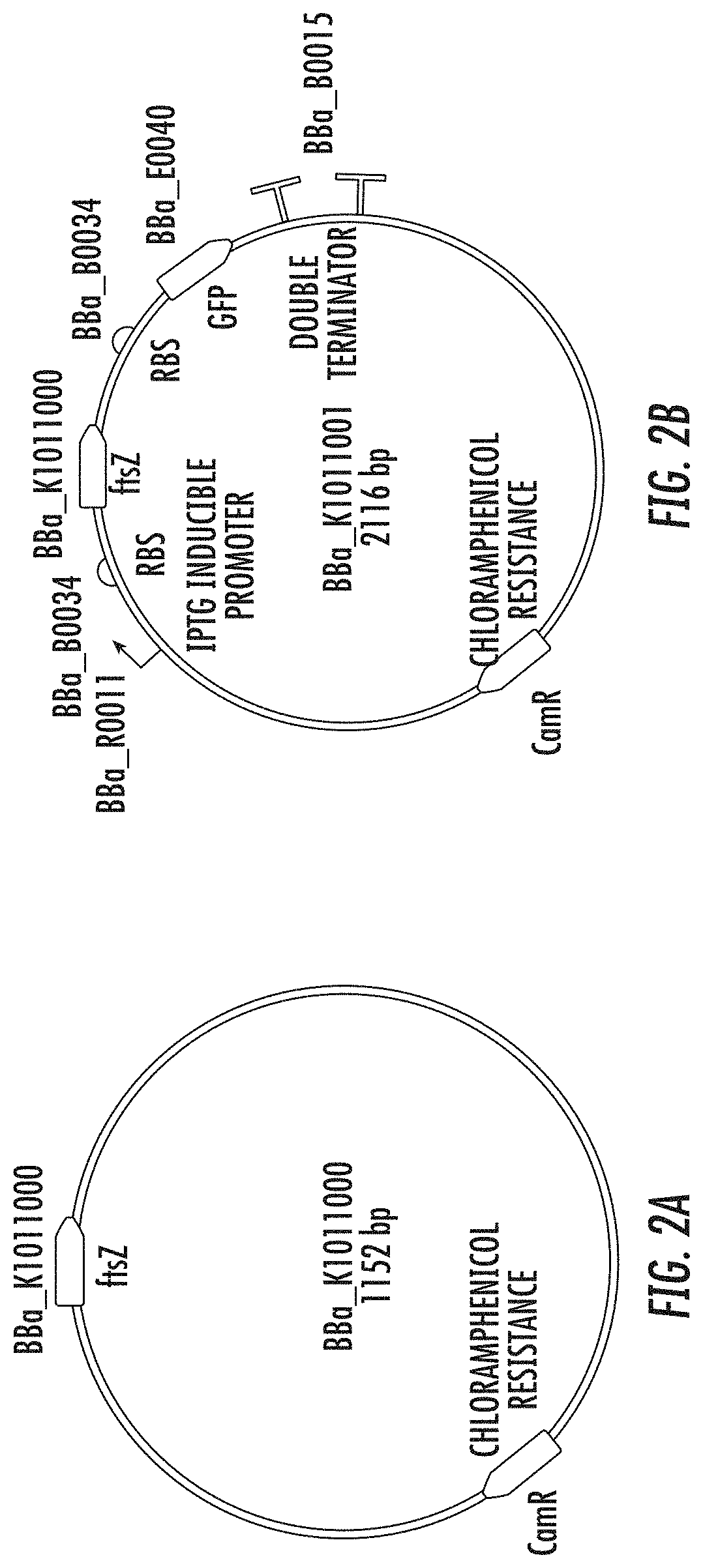
[0052]Construction of an IPTG-Inducible ftsZ Operon for the Production of E. coli Minicells
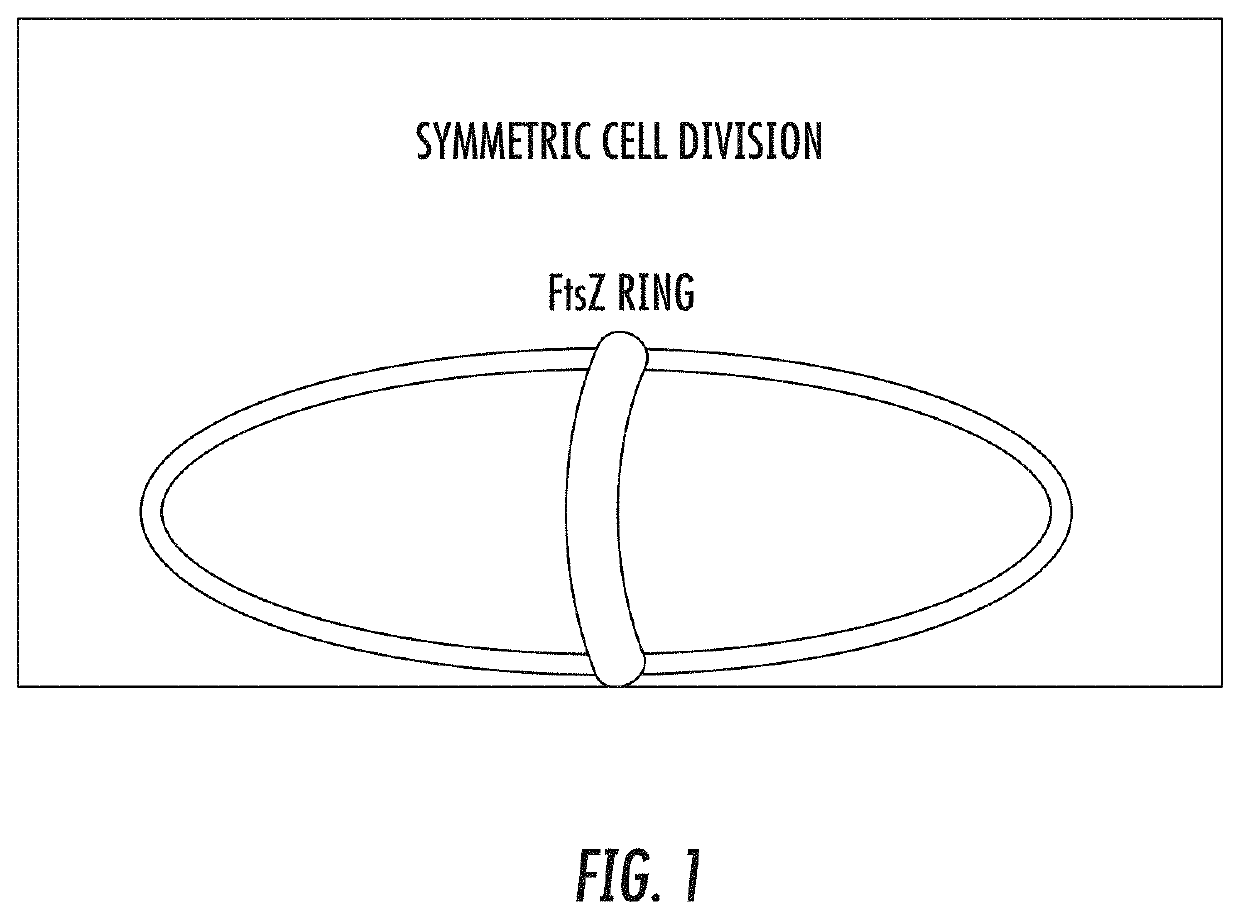
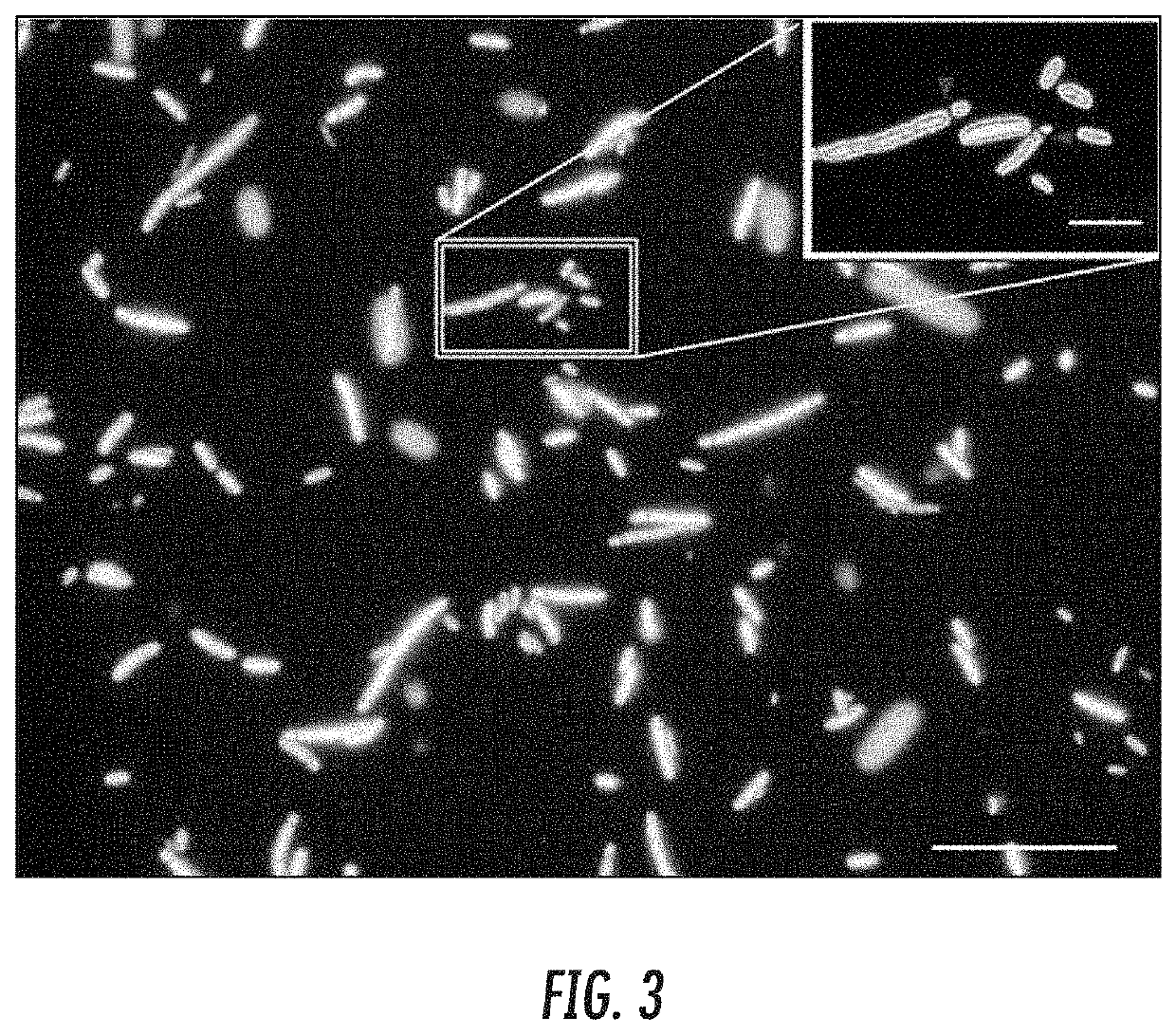
[0053]Despite many attractive qualities for synthetic biology, Escherichia coli and other engineering-amenable bacteria suffer from a number of inherent biosafety issues that limit their practical application. A safer alternative exists in the little-known nano-chassis called a minicell—the anucleoid product of an aberrant, asymmetric cell division. To introduce minicells to synthetic biology, a minicell-inducing operon from standardized parts was engineered. Through IPTG-tunable overexpression of the tubulin-homolog FtsZ, this operon enables optimization of E. coli minicell production. Whereas a spatially oscillating “Z ring” composed of FtsZ protein marks cleavage at the cell midline in wild type E. coli, overexpression of FtsZ initiates Z-ring assembly distal to the midline to form a minicell (FIG. 1). These engineered minicells are achromosomal bacterial particles that are unable to replic...
example 2
[0061]Plasmid Construct for Surface Expression of Organophosphate Hydrolase (OPH) on Minicells
[0062]An exemplary plasmid construct for surface expression of organophosphate hydrolase (OPH) on minicells is shown in FIGS. 7A-7B. The Knr designation represents the kanamycin resistance gene used in order to ascertain whether isolated minicells expressed OPH; any minicell lacking the plasmid would die upon exposure to sufficient levels of kanamycin. The PAllacO-1 designation represents the lac promoter that regulates the expression of our INPNC-OPH protein. In the absence of IPTG, expression of the INPNC-OPH protein will be actively repressed until IPTG is in solution. The p15A represents the origin of replication of the plasmid. This allows for the plasmid to be copied within the cell in order to ensure that maximal expression of the desired protein—INPNC-OPH—upon induction with IPTG. The INPNC-OPH gene codes for organophosphate hydrolase fused to an ice nucleation protein. The ice nucl...
example 3
[0063]Induction and Purification of Minicells
[0064]Bacterial strains that naturally produce minicells (commonly at a 2:1 ratio (2 bacterial cells for every one minicell) were used to obtain OPH-expressing minicells (produced using the construct shown in FIGS. 7A-7B and described in Example 2). Minicells were purified by differential centrifugation at 2,000 g for 10 min at 4° C. to pellet the parent bacteria followed by centrifugation of the supernatant at 10,000 g for 10 min at 4° C. to pellet minicells. The minicell pellet was then re-suspended in 50 mL LB and incubated at 37° C., 180 rpm for 90 min with 100 mg / L ceftriaxone. This dose of ceftriaxone is sufficient to cause cell lysis without having any detrimental effect on minicell integrity. The resulting minicell preparation was filtered at room temperature through a 0.45 μm dead end filter (Millipore SE1M003M00) to remove any remaining parent cells, followed by additional filtration with a 0.22 μm cross-flow filter (Millipore G...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| adhesion | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com