Methods of Treating Rheumatoid Arthritis
a technology for rheumatoid arthritis and treatment methods, applied in the field of treatment methods of rheumatoid arthritis, can solve the problems of a substantial number of patients that either fail to respond to treatment options or eventually become refractory to treatment, and achieve the effects of less frequent injections, less cost to patients, and increased patient complian
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Kinetic Analysis of Binding of Anti-CD22 Antibodies to CD22 and Surrogate Antigens / Anti-Idiotype Antibodies
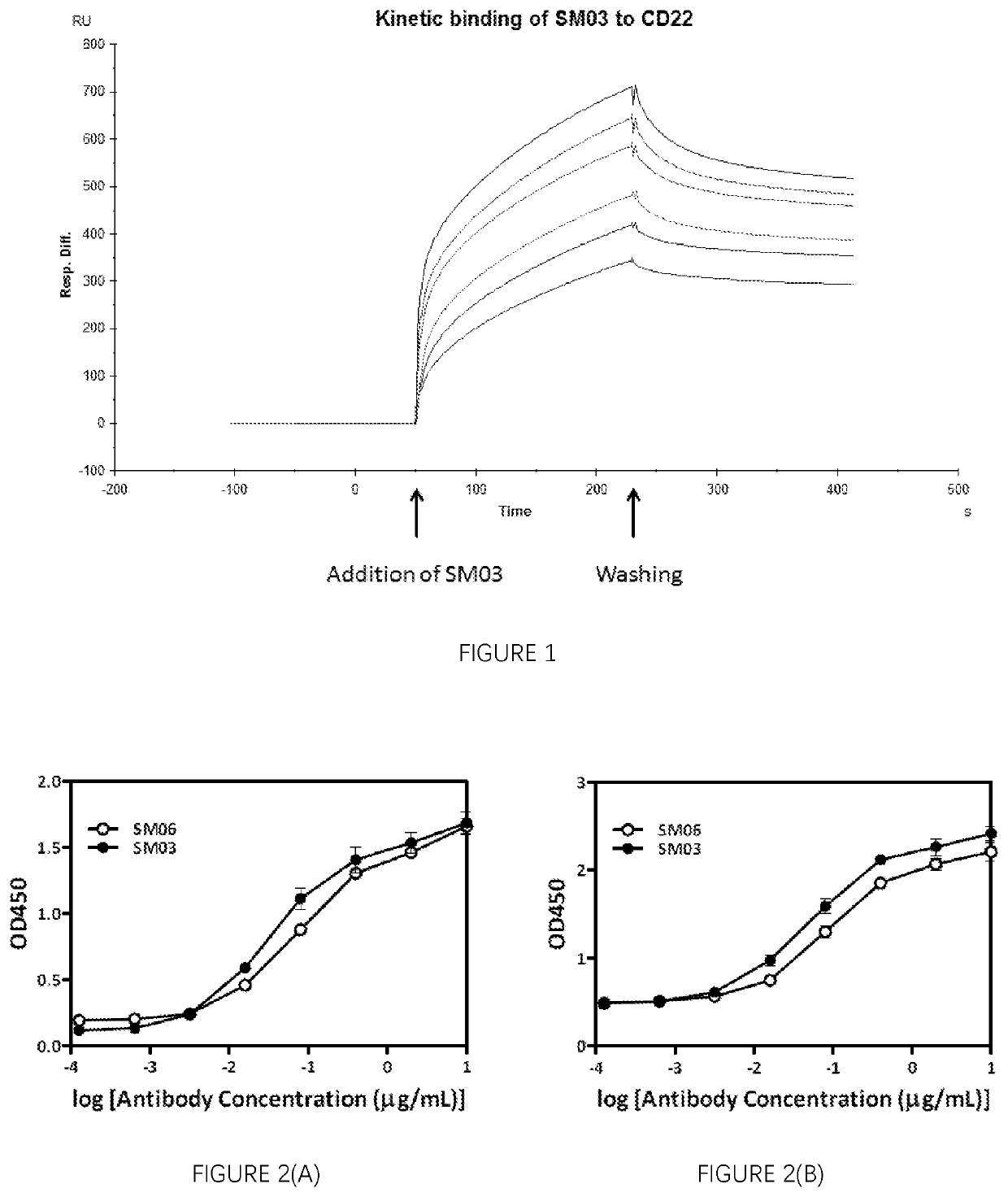
[0269]Affinity of anti-CD22 antibodies against human CD22 was performed using BIAcore (GE Healthcare Life Sciences, Piscataway, N.J.) according to standard procedures. Briefly, human recombinant CD22 (20 μg / ml, PeproTech, Rocky Hill, N.J.) was immobilized on carboxymethylated dextran-coated CM5 sensor chip. The chip surface was firstly activated with N-ethyl-N′-(3-diethylaminopropyl) carbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS). Different concentrations of anti-CD22 antibodies, and in this case, SM03 (1.0625-34 nmol / ml) in phosphate buffer saline (PBS) were injected at a flow rate of 20 μL / min for 3 min. The residual carboxyl groups were subsequently blocked by 1M ethanolamine (pH 8.5). The dissociation was studied by washing with running buffer for 3 min. The chip surface was then regenerated by injection with 50 mM glycine-CI for 60 s. Kinetic parameters in...
example 2
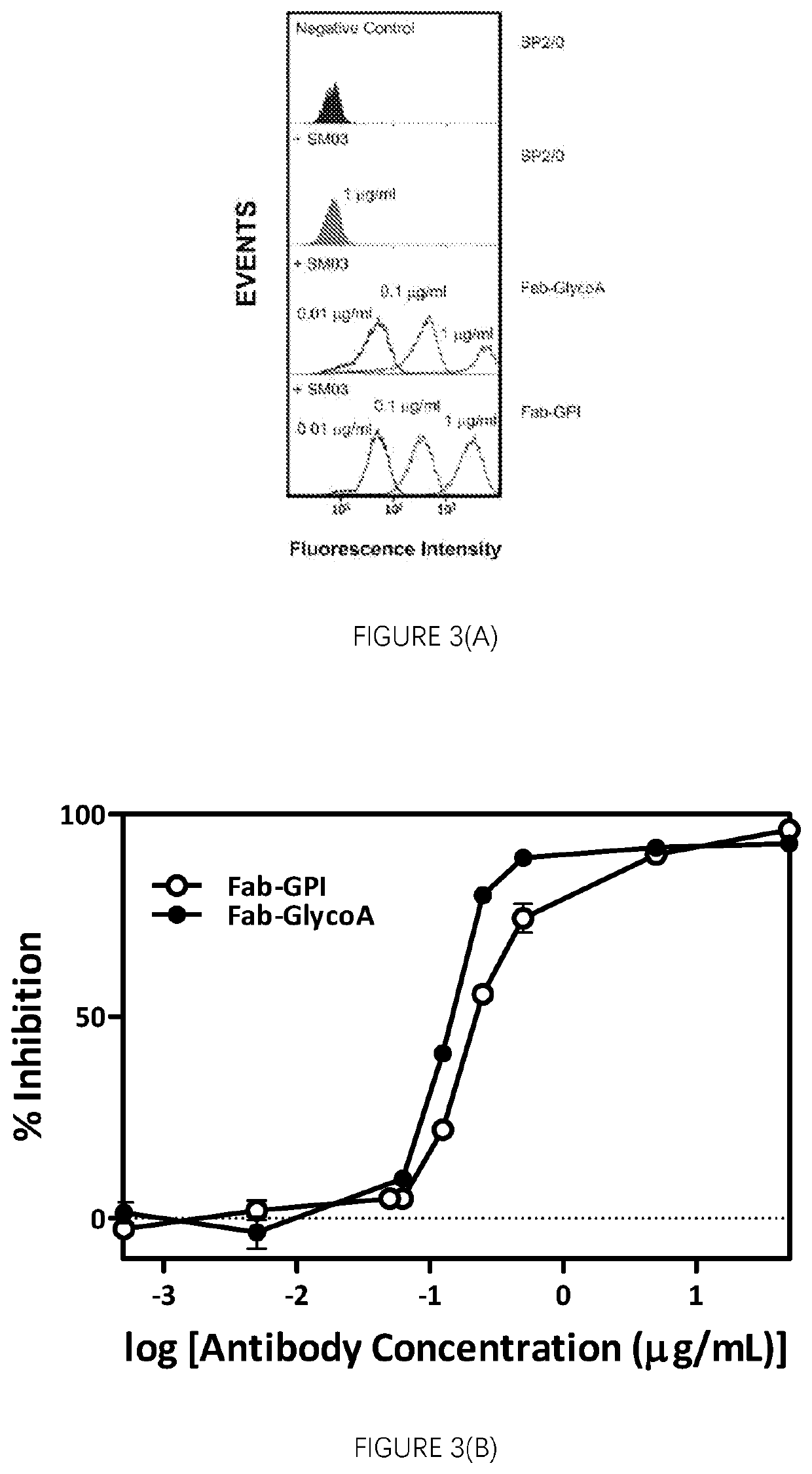
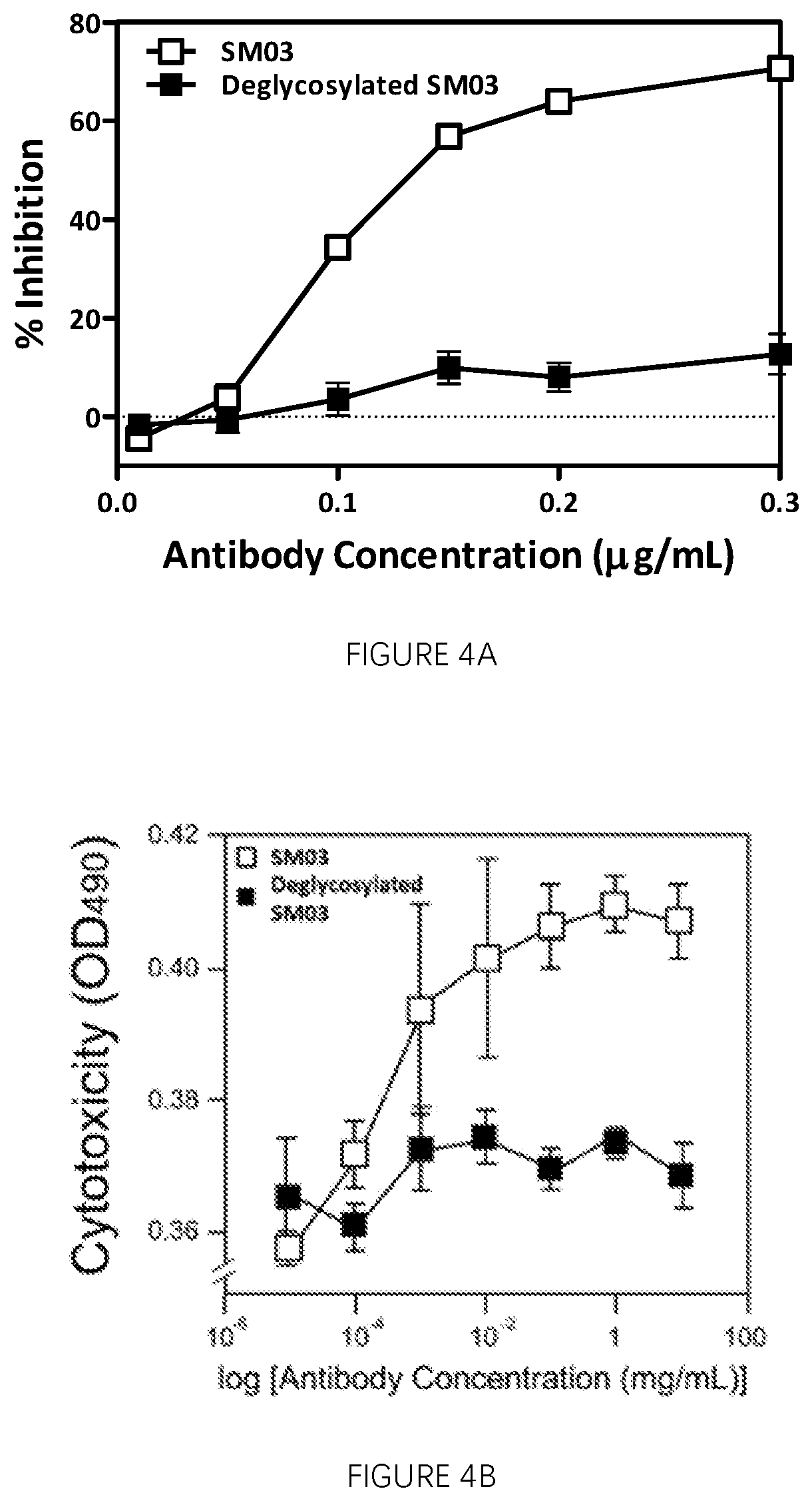
[0272]Functional Activities of Anti-CD22 Antibodies Against Surrogate Target Cell Lines Expressing on the Cell Surface with a Binding Moiety Specific for the Anti-CD22 Antibodies
[0273]FIG. 3 shows the murine myeloma SP2 / 0 cells transfected with expression vector encoding the Fab fragment of the anti-idiotype antibody against SM03 (LRID Fab) fused either to the transmembrane portion of Glycophorin A (Fab-GlycoA) or the glycophosphatidylinosito (GPI) signal sequence from decay accelerating factor (DAF) protein demonstrated high level of surface expression of LRID Fab (FIG. 3A) and these cells can be used as the surrogate target cells for the induction of CMC inhibition by the anti-CD22 antibodies SM03 and SM06. Exemplary SM03-induced CMC killing against the Fab-GlycoA and Fab-GPI surrogate target cell is shown (FIG. 3B). SP2 / 0 murine myeloma cells that express endogenous Igβ were transfected with vectors containing sequences encoding LRID Fab fused to either the transmembrane portion ...
example 3
Specific Binding Epitope Mapping for Anti-CD22 Antibodies
[0275]Preliminary epitope mapping was done by expressing different combinations of extracellular domains encompassing the human CD22 sequence in bacteria and the refolded recombinant proteins were tested for binding to SM03 in an ELISA assay. Only the refolded and purified human CD22 encompassing domain 2-4 (CD22d2-4) (SEQ ID 011) showed binding. The recombinant protein CD22d2-4 was analyzed by reducing SDS-PAGE (FIG. 5A), and ELISA plate coated with CD22d2-4 showed dose-response binding to the anti-CD22 antibody SM03 (FIG. 5B), indicating that the epitope resides within the domain 2-4 region of the human CD22 protein. There are 6 extracellular domains for human CD22 antigen that are expressed on the surface of matured B cells. Preliminary mapping studies by expressing different extracellular portions of human CD22 indicated that SM03 binds to the extracellular portion of human CD22 at regions encompassing domains 2-4 (CD22d2-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| ka | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com