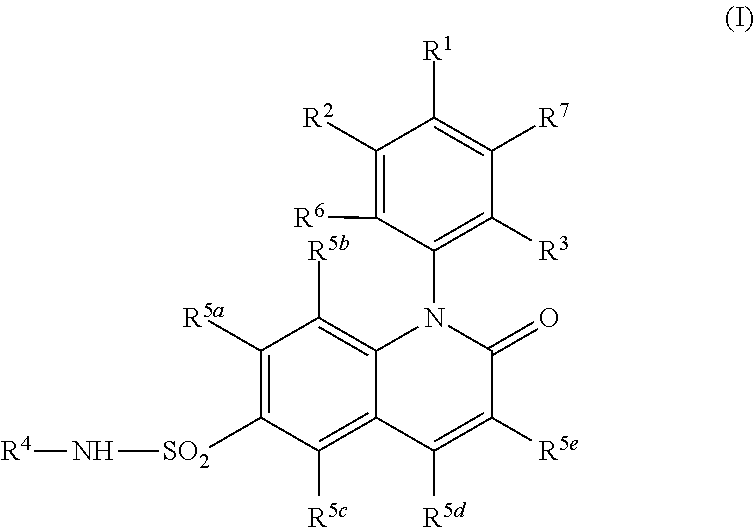
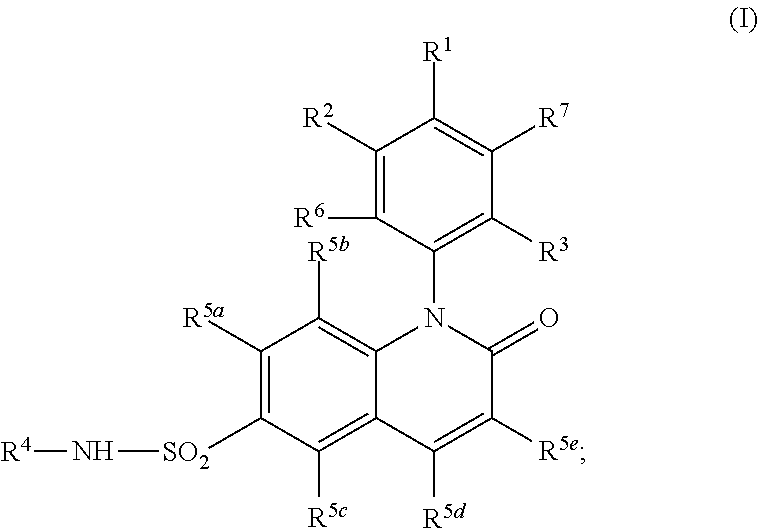
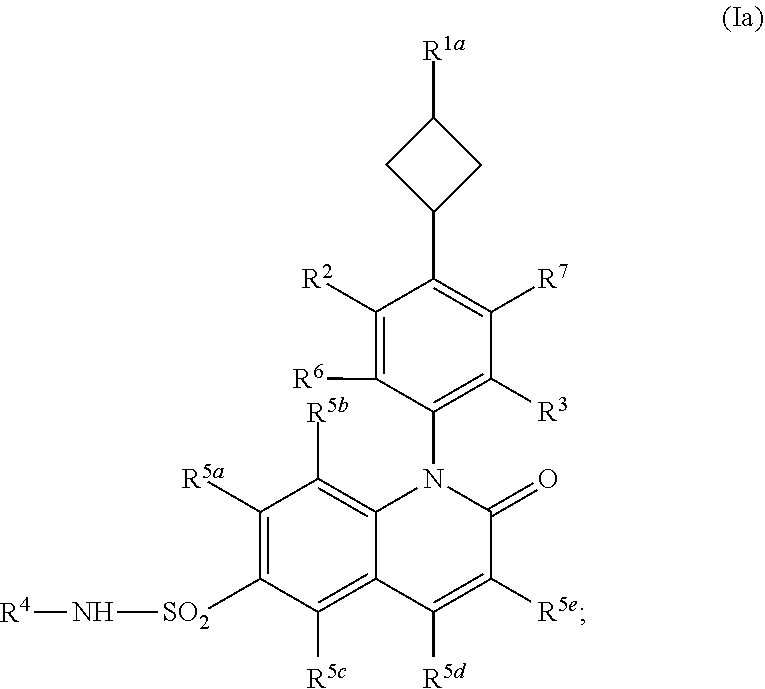
Cyclobutyl dihydroquinoline sulfonamide compounds
a technology of cyclobutyl dihydroquinoline and sulfonamide, which is applied in the field of cyclobutyl dihydroquinoline compounds, can solve the problems of nonselective sodium channel inhibitors, limited dose and use, and anxiety and over excitability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples 1 & 2
ert-Butyl)Cyclobutyl)-5-Fluoro-2-Methoxyphenyl)-N-(Isoxazol-3-Yl)-2-Oxo-1,2-Dihydroquinoline-6-Sulfonamide and 1-(4-(3-(Tert-Butyl)Cyclobutyl)-5-Fluoro-2-Methoxyphenyl)-N-(Isoxazol-3-Yl)-2-Oxo-1,2-Dihydroquinoline-6-Sulfonamide, Respectively
[0421]
STEP 1: (M)-1-(4-(3-(TERT-BUTYL)CYCLOBUTYL)-5-FLUORO-2-METHOXYPHENYL)-N-(ISOXAZOL-3-YL)-2-OXO-1,2-DIHYDROQUINOLINE-6-SULFONAMIDE
[0422]A vial was charged with palladium(II) acetate (2.7 mg, 0.012 mmol), 2′-(dicyclohexylphosphino)-N2,N2,N6,N6-tetramethyl-[1,1′-biphenyl]-2,6-diamine (10.6 mg, 0.024 mmol), and (M)-1-(4-bromo-5-fluoro-2-methoxyphenyl)-N-(isoxazol-3-yl)-2-oxo-1,2-dihydroquinoline-6-sulfonamide (0.100 g, 0.202 mmol). (3-(tert-butyl)cyclobutyl)zinc(II) iodide (0.2 M in THF, 2.0 mL, 0.41 mmol) was added and the reaction was stirred for two hours at 50° C. The reaction was then diluted with ethyl acetate and washed twice with 1 N HCl. The organic layer was washed with brine, dried with sodium sulfate, filtered, and concentrated. The ...
example 3
(3-(Tert-Butyl)Cyclobutyl)-5-Fluoro-2-Methoxyphenyl)-N-(Isoxazol-3-Yl)-2-Oxo-1,2-Dihydroquinoline-6-Sulfonamide
[0424]
[0425]A vial was charged with palladium(II) acetate (7.3 mg, 0.032 mmol) 2′-(dicyclohexylphosphino)-N2,N2,N6,N6-tetramethyl-[1,1′-biphenyl]-2,6-diamine (28 mg, 0.065 mmol), and (P)-1-(4-bromo-5-fluoro-2-methoxyphenyl)-N-(isoxazol-3-yl)-2-oxo-1,2-dihydroquinoline-6-sulfonamide (160 mg, 0.324 mmol). (3-(tert-Butyl)cyclobutyl)zinc(II) iodide (0.2 M in THF, 3.2 mL, 0.65 mmol) was added, and the reaction was stirred for two hours at 50° C. The reaction was then diluted with ethyl acetate and washed twice with 1 N HCl. The organic layer was washed with brine, dried with sodium sulfate, filtered, and concentrated. The material was purified via column chromatography (RediSep Gold 40 g column, gradient elution 0-50% [3:1 EtOAc:EtOH]:heptane) to give (P)-1-(4-(3-(tert-butyl)cyclobutyl)-5-fluoro-2-methoxyphenyl)-N-(isoxazol-3-yl)-2-oxo-1,2-dihydroquinoline-6-sulfonamide (90 mg, ...
example 4
(3,3-Difluorocyclobutyl)-5-Fluoro-2-Methoxyphenyl)-N-(Isoxazol-3-Yl)-2-Oxo-1,2-Dihydroquinoline-6-Sulfonamide
[0426]
STEP 1: (P)-1-(5-FLUORO-2-METHOXY-4-(5,8-DIOXASPIRO[3.4]OCTAN-2-YL)PHENYL)-N-(ISOXAZOL-3-YL)-N-(4-METHOXYBENZYL)-2-OXO-1,2-DIHYDROQUINOLINE-6-SULFONAMIDE
[0427]A vial was charged with (P)-1-(4-bromo-5-fluoro-2-methoxyphenyl)-N-(isoxazol-3-yl)-N-(4-methoxybenzyl)-2-oxo-1,2-dihydroquinoline-6-sulfonamide (Intermediate F) (0.500 g, 0.814 mmol), palladium(II) acetate (10.96 mg, 0.049 mmol), and 2′-(dicyclohexylphosphino)-N2,N2,N6,N6-tetramethyl-[1,1′-biphenyl]-2,6-diamine (0.043 g, 0.098 mmol). 5,8-Dioxaspiro[3.4]octan-2-ylzinc(II) bromide (0.1 M in THF, 14 mL, 0.70 mmol) was added and the reaction was stirred at 50° C. for 16 h. The reaction was then diluted with ethyl acetate and washed with water. The aqueous layer was extracted with ethyl acetate, and the combined organic layers were washed with brine, dried with sodium sulfate, filtered, and concentrated. The material w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com