Method of treating the syndrome of coronary heart disease risk factors in humans
a risk factor and human syndrome technology, applied in the field of human syndrome of coronary heart disease risk factors, can solve the problems of inability to correct the entire hyperlipidemic complex with a single therapeutic agent, the exact interrelationship between the disease states that make up these syndromes is not fully understood, and the lipid fraction of dyslipidemia may be predominan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
[0059]The example is being described for purely illustrative purposes, and is in no way meant to limit the scope of the invention.
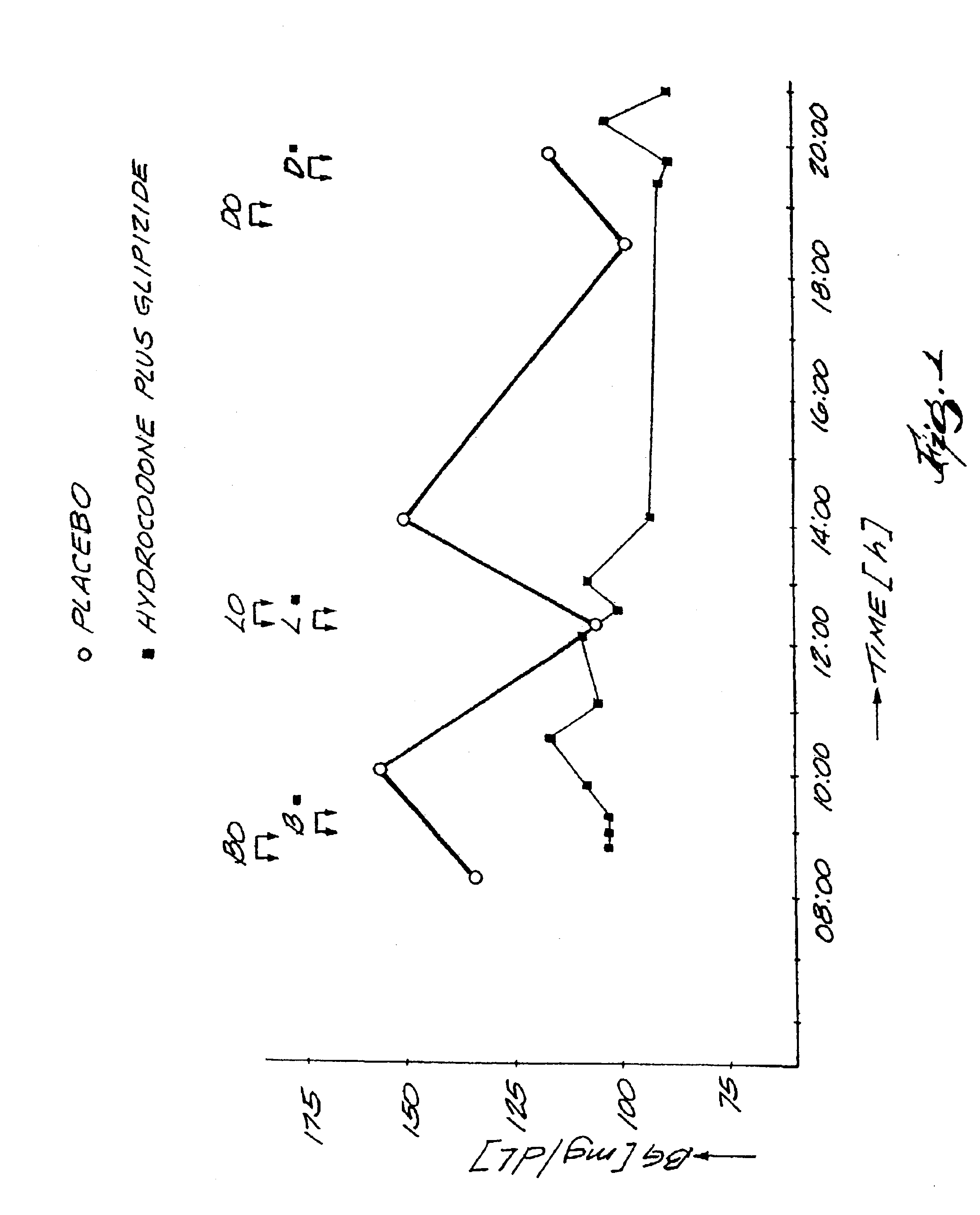
[0060]The daily blood glucose profile of a 72 year old subject with Type 2 Diabetes and dyslipidemia was monitored following the early morning oral administration of 0.15 mg Hydrocodone, a centrally acting μ-agonist in combination with sulfonylurea type insulin secretagogue. The glucose profile of the subject was monitored on the second day after the treatment had been initiated. Sufficient time was allowed after the earlier treatment to eliminate any carry-over effect.
Treatment 1
[0061](-O-) no drug treatment.
[0062]Breakfast: 08:40-09:00; lunch: 12:25-12:50; dinner: 18:40-19:00
[0063]
time [h]08:1010:0012:2014:0018:3020:00BG [mg / dL]13215610415098117
Treatment 2
[0064](-▪-) 0.15 mg Hydrocodone, a centrally acting μ-agonist in combination with 1.0 mg Glipizide, administered 2 hours before breakfast.
[0065]Breakfast: 09:00-0915; lunch: 12:10-12:30; dinner: 19:20-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Dimensionless property | aaaaa | aaaaa |
| Electrical resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com