Organopolysiloxanes containing phosphonic groups, method for the production and use thereof
a technology of organopolysiloxanes and phosphonic acid groups, which is applied in the preparation of carboxylic compounds, ester-hydroxy reaction preparations, cation exchangers, etc., can solve the problems of loss of active functional groups, large amount of bi-products and waste, and waste treatment and destruction of unwanted products and wastes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
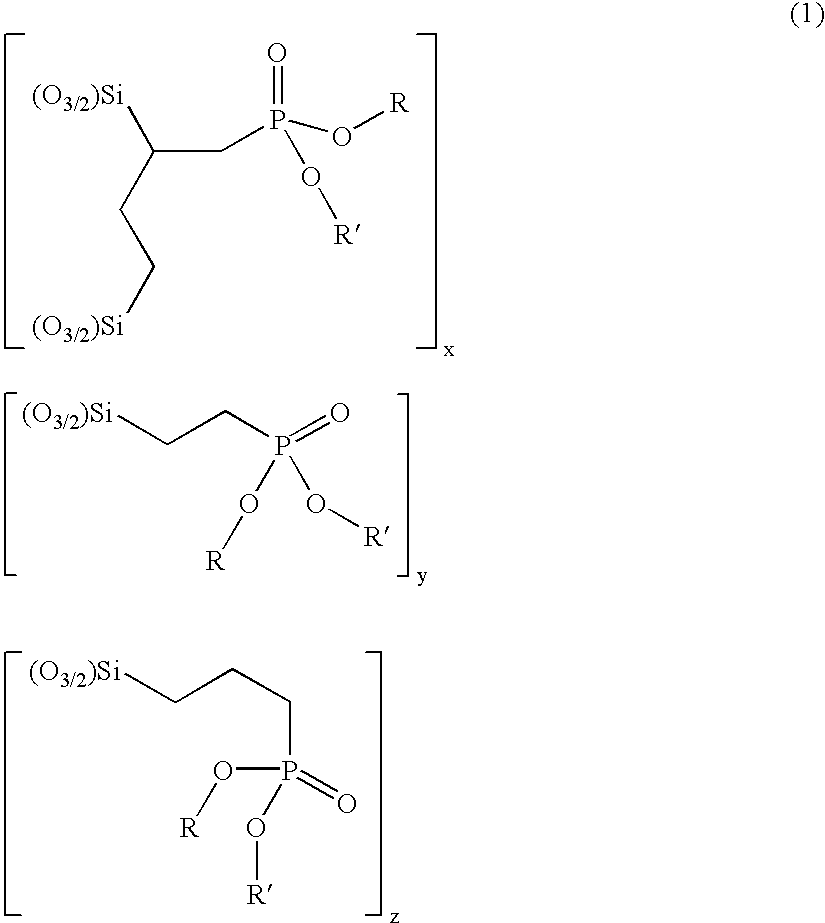
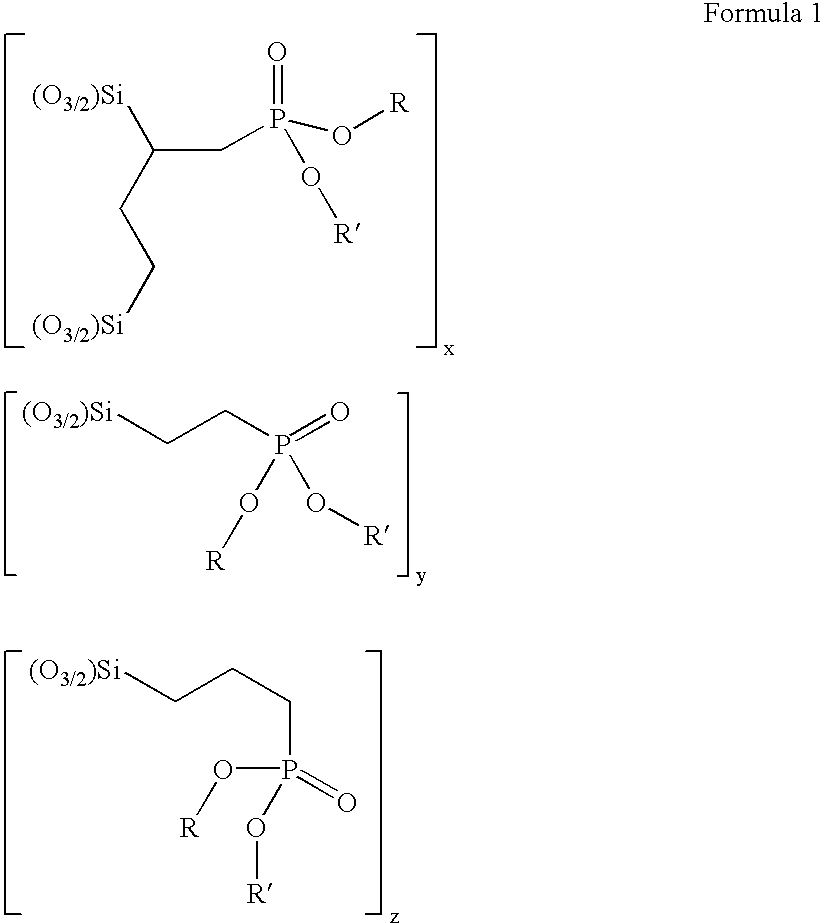
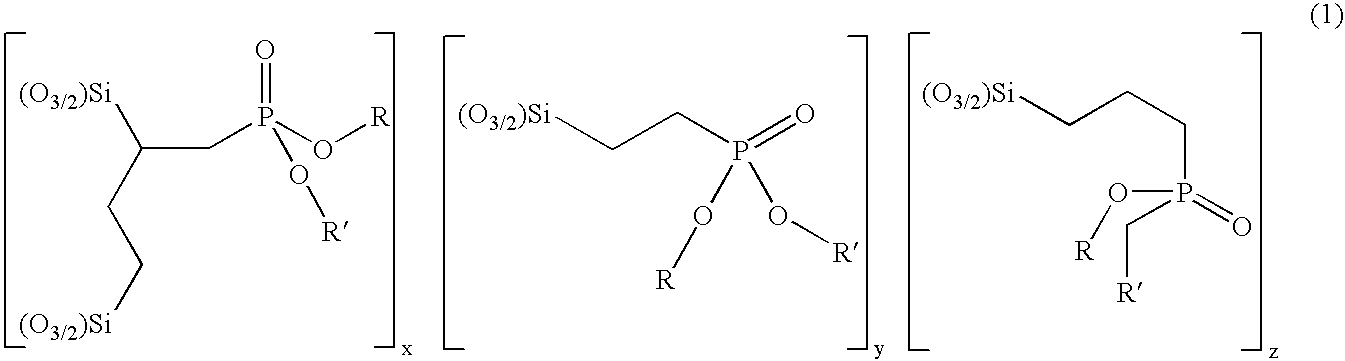
[0111]A solution containing triethoxy vinyl silane (19.0 g, 0.136 mol), diethyl phosphite (19.32 g, 0.136 mol) and di-tertbutyl peroxide (6 drops) was heated at 120–130° C. under an atmosphere of nitrogen. Heating was continued for 40 h and di-tert butyl peroxide (6 drops) was added every 4 h. Un-reacted starting material was removed by heating at 120° C.—bath temperature—under reduced pressure (2 mm Hg) to give a mixture of diethyl 2,4di(triethoxysilyl)butylphosphonate and diethyl 2-triethoxysilyl ethylphosphonate as a colourless oil (30.1 g) in a ratio of 1.8:3.2.
[0112]The oil (30.1 g) was dissolved in methanol (125 ml) containing 1M HCl (10 ml). The solution was left at ambient temperature for 48 h and then at 55° C. for 100 h. The resultant glass (16.0 g) was crushed and then added to concentrated HCl (150 ml). The mixture was gently refluxed with stirring for 10 h and then cooled to room temperature. The solid was filtered and first washed with distilled water till the washings...
example 2
[0115]A solution containing triethoxy vinyl silane (38.8 g, 0.204 mol), diethyl phosphite (28.17 g, 0.204 mol) and di tertbutyl peroxide (6 drops) was heated at 120–130° C. under an atmosphere of nitrogen. Heating was continued for 40 h and di-tert butyl peroxide (6 drops) was added every 4 h. Un-reacted starting material was removed by heating at 120° C.—bath temperature—under reduced pressure (2 mm Hg) to give a mixture of diethyl 2,4-di(triethoxysilyl)butylphosphonate and diethyl 2-triethoxysilyl ethylphosphonate as a colourless oil (55.1 g) in a ratio of 1.1:1.8.
[0116]The oil (55.1 g) was dissolved in methanol (200 ml) and then 1M HCl (20 ml) was added with stirring. The solution was left at ambient temperature for 48 h and then at 55° C. for 100 h. The resultant glass (30.0 g) was crushed and then added to concentrated HCl (300 ml). The mixture was gently refluxed with sting for 10 h and then cooled to room temperature. The solid was filtered and first washed with distilled wat...
example 3
[0117]A solution containing triethoxy vinyl silane (38.8 g, 0.204 mol), diethyl phosphite (42.25 g, 0.306 mol) and di-tertbutyl peroxide (6 drops) was heated at 120–130° C. under an atmosphere of nitrogen. Heating was continued for 40 h and di tert butyl peroxide (6 drops) was added every 4 h. Un-reacted starting material was removed by heating at 120° C.—bath temperature—under reduced pressure (2 mm Hg) to give a mixture of diethyl 2,4-di(triethoxysilyl)butylphosphonate and diethyl 2-triethoxysilyl ethylphosphonate as a colourless oil (58.6 g) in a ratio of 1:2.1.
[0118]The oil (58.6 g) was dissolved in methanol (230 ml) and then 1M HCl (25 ml) was added with stirring. The solution was left at ambient temperature for 4 h and then at 55° C. for 200 h. The resultant glass (32.0 g) was crushed and then added to concentrated HCl (305 ml). The mixture was gently refluxed with stirring for 10 h and then cooled to room temperature. The solid was filtered and first washed with distilled wat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com