Manufacture of five-carbon sugars and sugar alcohols
a technology of five-carbon sugars and sugar alcohols, which is applied in the preparation of sugar derivatives, monosaccharides, enzymes, etc., can solve the problems of little effort in the opposite direction of modifying microorganisms and no method of further improving natives, so as to improve the spectrum of five-carbon carbohydrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Cloning of the B. subtilis rpi Gene Coding for D-Ribose-Phosphate Isomerase
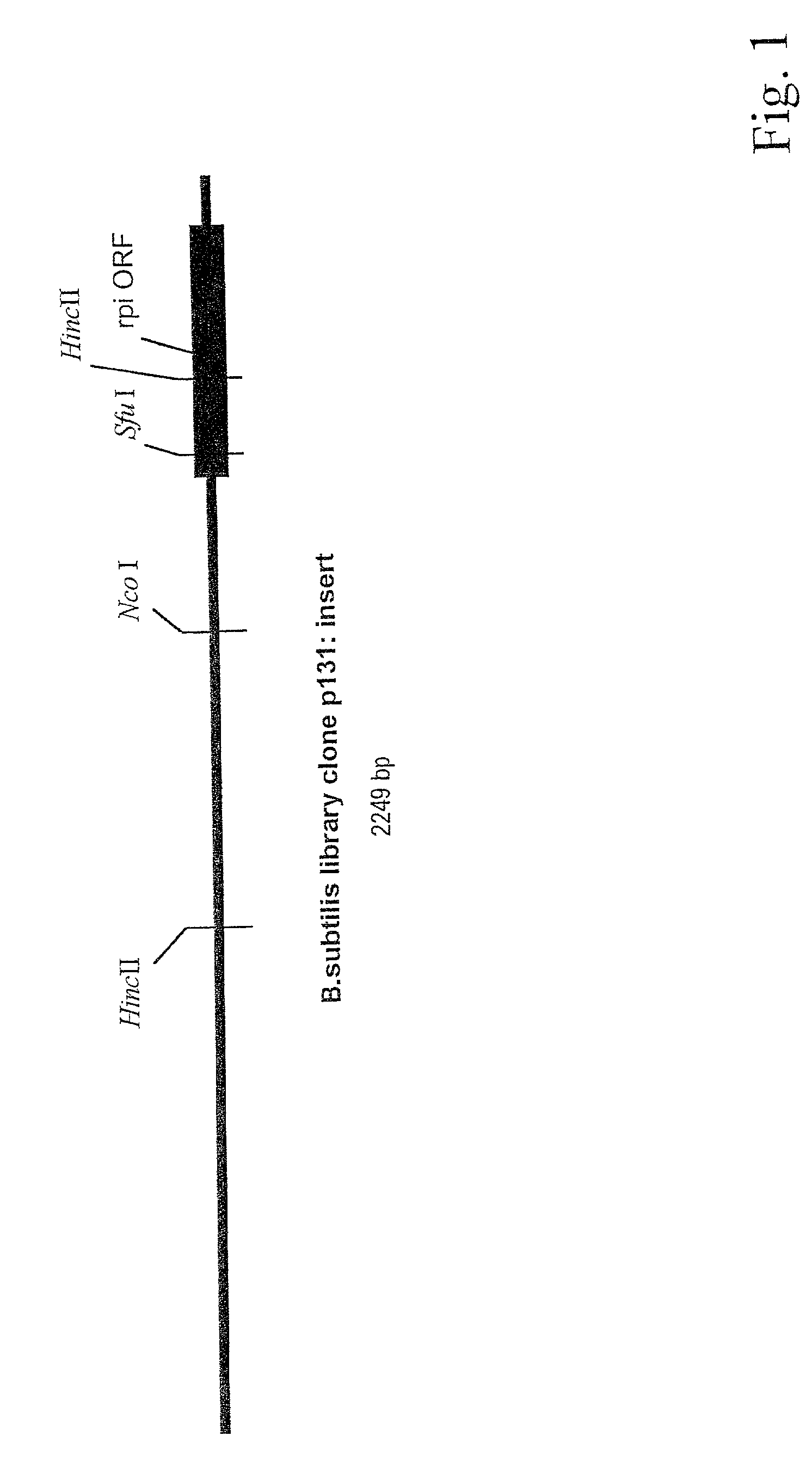
[0225]At the time when the work was initiated, the complete genomic sequence of B. subtilis was not yet available. Also, it was not known whether B. subtilis contained one or more D-ribose-phosphate isomerase genes (E. coli was known to contain two). Therefore, the strategy for cloning the rpi gene(s) was based on functional complementation of D-ribose-auxotrophic mutation in E. coli rather than on PCR. Presently, the preferred mode for cloning the rpi gene(s) would be to use PCR based techniques rather than the method as exemplified below. However, the method described in this example is fully adequate for practicing the present invention.
[0226]A gene library was constructed from the DNA of B. subtilis (ATCC 6051). The DNA of this strain was partially cut with the restriction endonuclease Sau3A and fragments exceeding 3 kb in size were isolated by preparative agarose gel electrophoresis. Unless indicated oth...
example 2
Construction of B. subtilis Strains Containing rpi− and tkt− Mutation
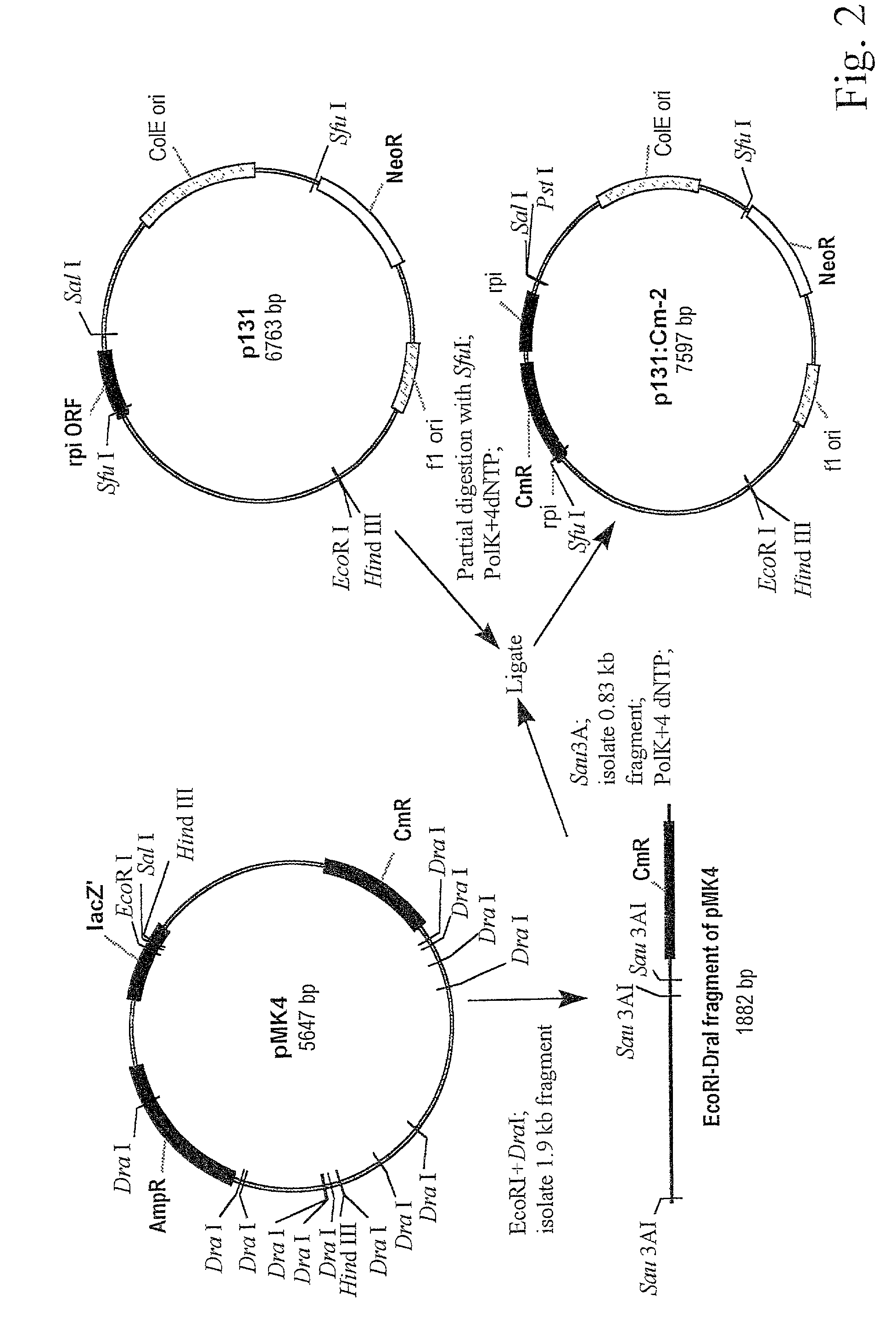
[0227]The chloramphenicol resistance gene was isolated from the plasmid pMK4 (obtained from the Bacillus Genetic Stock Center (BGSC, Ohio, USA). pMK4 was digested with DraI and EcoRI, a 1.9 kb fragment was isolated from the digest by preparative agarose gel electrophoresis and further digested with Sau3A. A purified 0.83 kb fragment from this digest was purified and treated with Klenow fragment of the DNA-polymerase I in the presence of all four deoxynucleotide triphosphates. This fragment was than ligated with the plasmid p131 (Example 1) digested with SfuI and similarly treated with the Klenow fragment. Plasmid p131-Cm2 containing the B. subtilis rpi gene disrupted by the chloramphenicol resistance gene was isolated after transformation of E. coli with this ligation mixture (FIG. 2).
[0228]p131-Cm2 was digested with EcoRI and PstI and the digest was used to transform the B. subtilis strain BD170 (trpC2, thr−5, obt...
example 3
Construction of D-ribulose-producing B. subtilis Strains
[0231]Chromosomal DNA was isolated from the strain GX1 by the method referred to in Example 2 (except that Na-sarcosyl used in the original protocol was replaced with Na-dodecylsulfate). This DNA was used to transform the D-ribose-producing B. subtilis strain 31094 (U.S. Pat. No. 3,970,522) using the natural competence based method (Example 2). The transformants were screened by the PCR methods described in the Example 2 and also by studying the products of the glucose fermentation by these strains. One clone generating the fragment of the expected size in PCR with the oligonucleotide pair oBS-RPI5 and oBS-RPI3 and retaining the ability of the parent strains to convert glucose into five-carbon sugars was selected. The rpi-disrupted derivative of strain ATCC 31094 was named GX2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| angle | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com