Nitrile hydratase variant
a nitrile hydratase and nitrile technology, applied in the field of new nitrile hydratase, can solve the problem that there is no attempt to modify the above-mentioned properties, and achieve the effect of improving the enzyme's original activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
reference example 1
Construction (1) of the Substituted Amino Acid Having Nitrile Hydratase Activity
[0210]For the substitution with Met for the 6th Leu in the α-subunit, introduction of site-specific mutation was performed using a “LA PCR in vitro mutagenesis Kit” (manufactured by Takara Shuzo Co., Ltd.). Hereinafter, the “LA PCR in vitro mutagenesis Kit” is simply referred to as the kit. In following Reference Examples, the kit was handled on the basis of the principle thereof and in accordance with the manufacturer's instructions for the kit.
[0211]10 ml of a liquid LB medium was put into a 30 ml test tube, and sterilized by autoclaving at 121° C. for 20 minutes. Ampicillin was added to this medium to have a final concentration of 100 μg / ml. On the medium, one platinum loop of the cells of MT-10822 were inoculated and incubated therein at 37° C. for about 20 hours with stirring at 300 rpm. 1 ml of the resulting culture was put into a suitable centrifugal tube, and this was subjected to centrifugation ...
reference example 2
Construction (2) of the Substituted Amino Acid Having Nitrile Hydratase Activity
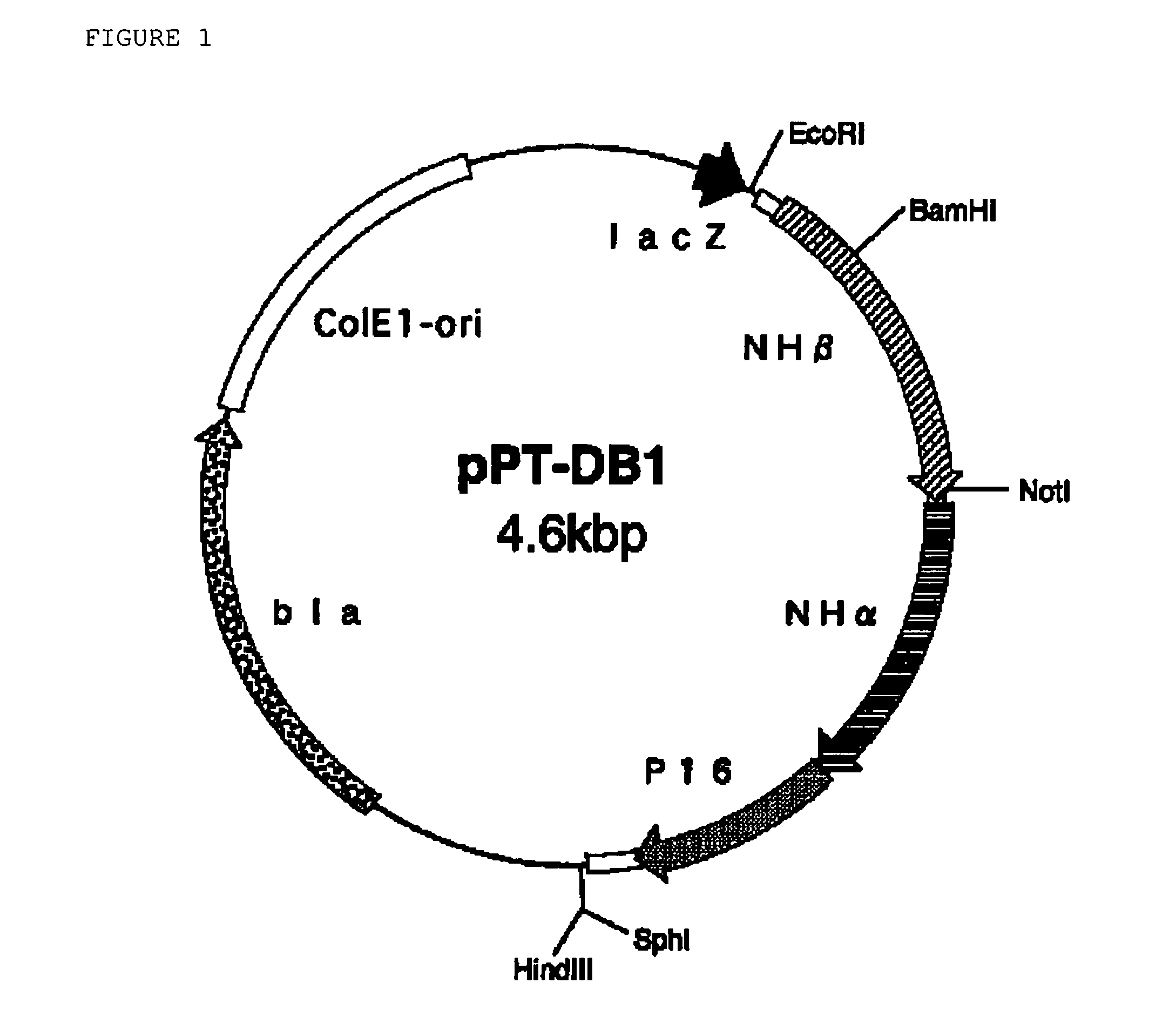
[0217]For the substitution with Thr for the 6th Leu in the α-subunit, using the plasmid DNA pPT-DB1 as the template, the plasmid DNA pPT-DB1 was subjected to introduction of site-specific mutation in the same manner as in Reference Example 1.
[0218]Using 10 ng of the plasmid DNA pPT-DB1 as prepared in Reference Example 1 as the template, PCRs of two different types were carried out. For the PCR reaction No. 1, a reaction system of 50 μl in total comprising 50 pmols of the primer having the sequence as forth in SEQ ID No: 11 in the Sequence Listing and 50 pmols of an M13 primer M4 (having the sequence as forth in SEQ ID No: 8 in the Sequence Listing) (for the composition of the system, the manufacturer's instructions for the kit were followed) was used, and the reaction consisted of 25 PCR cycles, in which one PCR cycle comprised thermal denaturation (98° C.) for 15 seconds, annealing (55° C.) for 30 secon...
reference example 3
Construction (3) of the Substituted Amino Acid Having Nitrile Hydratase Activity
[0222]For the substitution with Ala for the 6th Leu in the α-subunit, using the plasmid DNA pPT-DB1 as the template, the plasmid DNA pPT-DB1 was subjected to introduction of site-specific mutation in the same manner as in Reference Example 1.
[0223]Using 10 ng of the plasmid DNA pPT-DB1 as prepared in Reference Example 1 as the template, PCRs of two different types were carried out. For the PCR reaction No. 1, a reaction system of 50 μl in total comprising 50 pmols of the primer having the sequence as forth in SEQ ID No: 12 in the Sequence Listing and 50 pmols of an M13 primer M4 (having the sequence as forth in SEQ ID No: 8 in the Sequence Listing) (for the composition of the system, the manufacturer's instructions for the kit were followed) was used, and the reaction consisted of 25 PCR cycles, in which one PCR cycle comprised thermal denaturation (98° C.) for 15 seconds, annealing (55° C.) for 30 secon...
PUM
| Property | Measurement | Unit |
|---|---|---|
| radius | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| freezing point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com