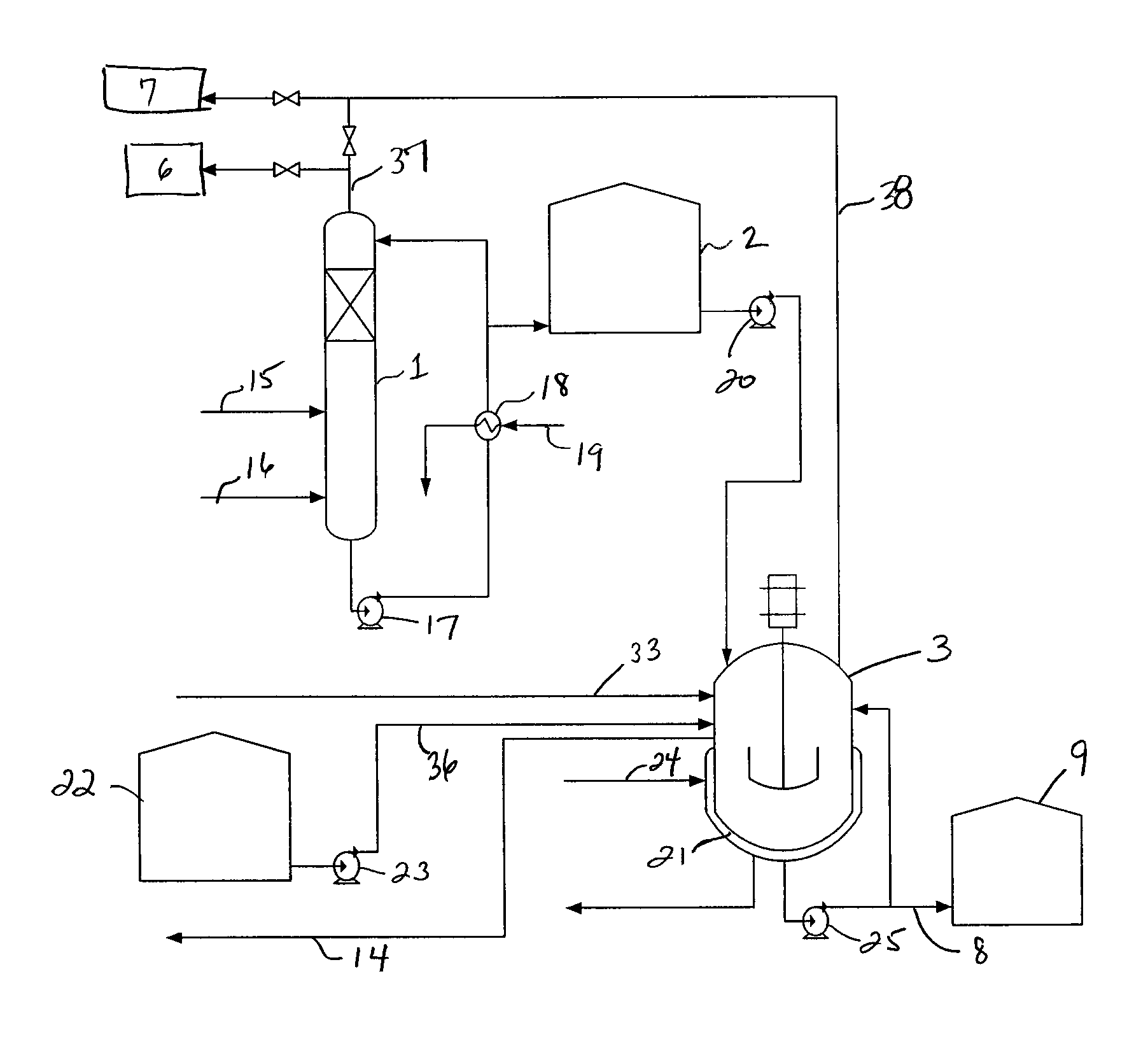
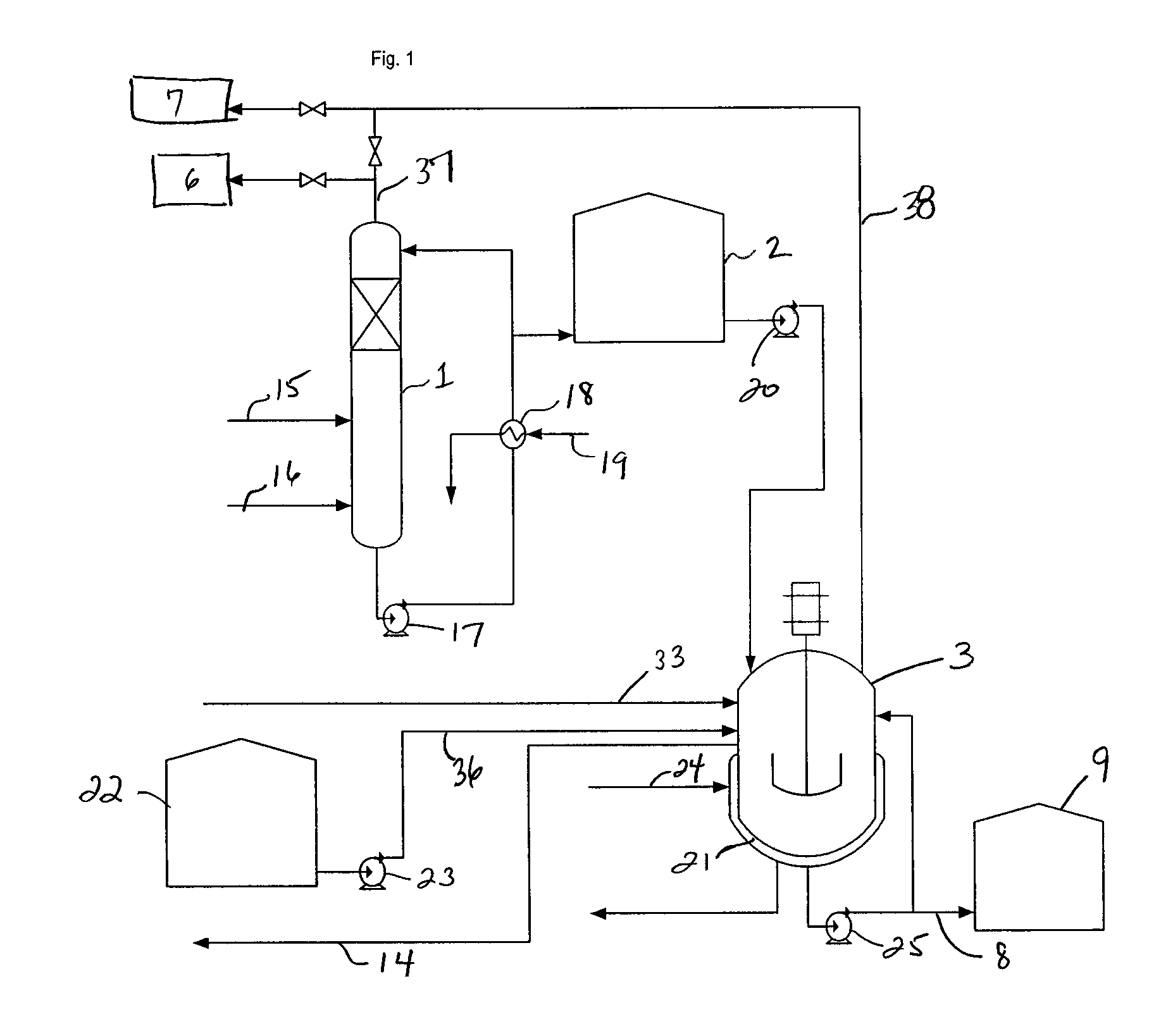
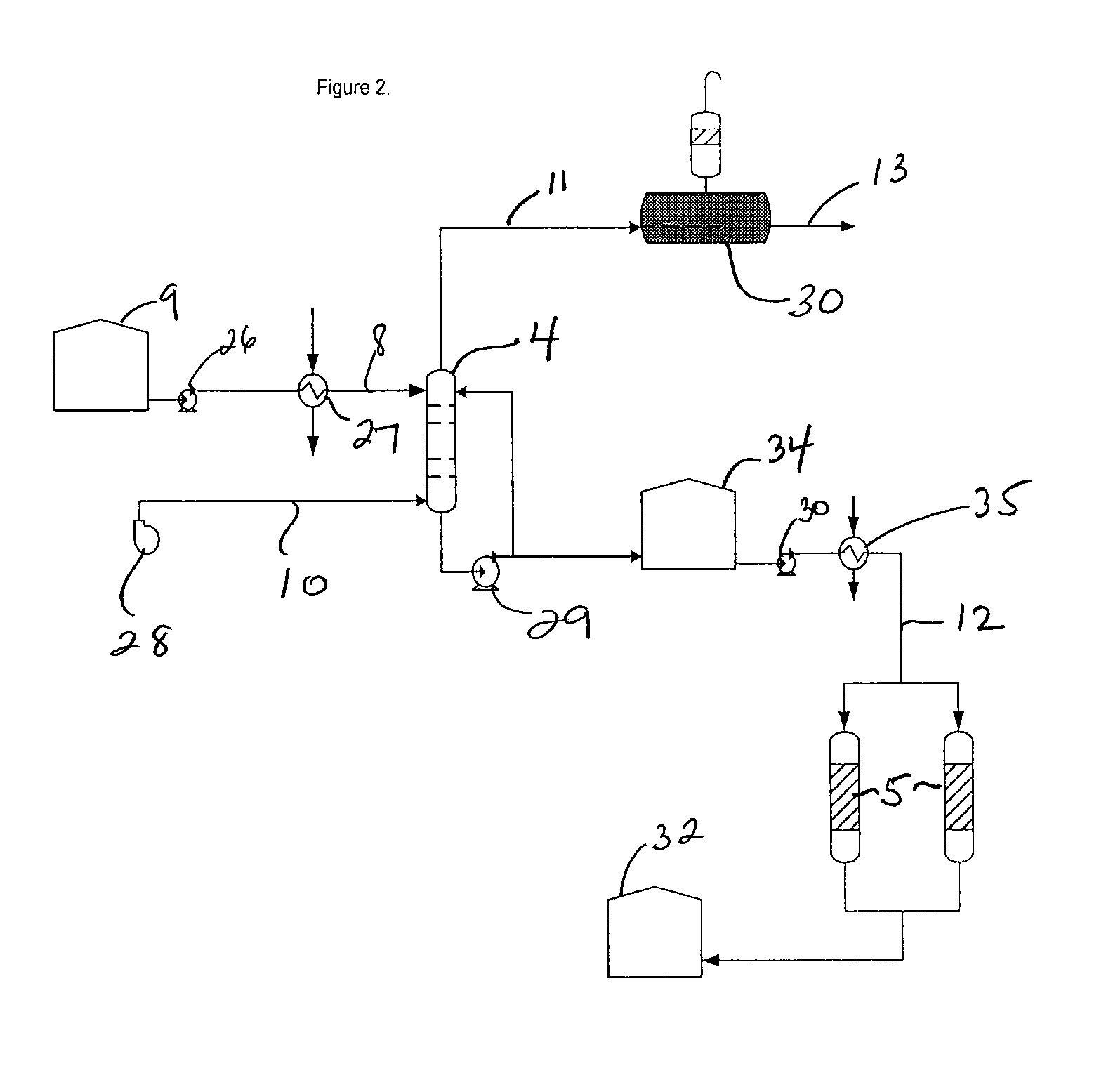
Process for conversion of waste fluid streams from chemical processing plants to beneficiary agriculture products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Treatment of Ammonium Sulfide with Virgin Sulfuric Acid
[0076]A 2 liter five neck round bottom flask equipped with mechanical agitator, condenser, thermometer, and two addition funnels for sulfuric acid and ammonium sulfide solution (ASD) was used. 100 grams of 40% commercial ammonium sulfate (AST) was added to the 2 liter flask as a starter solution heel. The top of the condenser exited directly to a caustic scrubber containing 962 grams of 20% caustic soda. The sulfuric acid addition funnel contained 596 grams of the 65% acid. The ammonium sulfide addition flask contained 520 ml of 45% ammonium sulfide solution. The mole ratio of virgin acid to ASD was 1.15 to 1.0. 180 grams of water was added to adjust the product AST concentration to 40%. ASD and virgin acid were dropped into the agitated ammonium sulfate solution. Temperature rose from 25° C. at the beginning of reaction to about 50° C. at the end of reaction. The crude ammonium sulfate product weight was 864.6 grams of colorles...
example 2
Treatment of Ammonium Sulfide with Spent Alkylation Acid and a Heel or Initial Charge of AST
[0079]A 2 liter five neck round bottom flask equipped with mechanical agitator, condenser, thermometer, and two addition funnels for spent sulfuric acid and ammonium sulfide solution (ASD) was used. 100 grams of 40% ammonium sulfate (AST) solution was introduced to the flask prior to the introduction of the acid or ASD. The condenser exit was directed to a caustic scrubber containing 550 grams of 20% caustic soda. The spent sulfuric acid addition funnel contained 452 ml of 65% spent acid. The ammonium sulfide addition flask contained 472 ml of 49.6% ammonium sulfide solution. The mole ratio of spent acid to ASD was 1.15 to 1.0. 180 grams of water was added to adjust the product AST concentration to 40%. ASD and spent acid were dropped to the agitated ammonium sulfate solution. Temperature rose from 25° C. at the beginning of reaction to about 50° C. at the end of reaction. The crude ammonium ...
example 3
Treatment of Ammonium Sulfide with Sulfuric Acid with Cooling the Ammonium Sulfate Reactor
[0083]The above reactions were repeated without the initial charge of 40% ammonium sulfate (AST) solution, but the ammonium sulfide (ASD) was placed in the reactor along with the required water amount to produce 40% ASD solution. The solution was cooled to about 5-15° C. and sulfuric acid was added at a rate that the exiting hydrogen sulfide gas was completely absorbed into the caustic scrubber with no breakthrough. The reaction / production time was cut from about 3 hrs to about 30 minutes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com