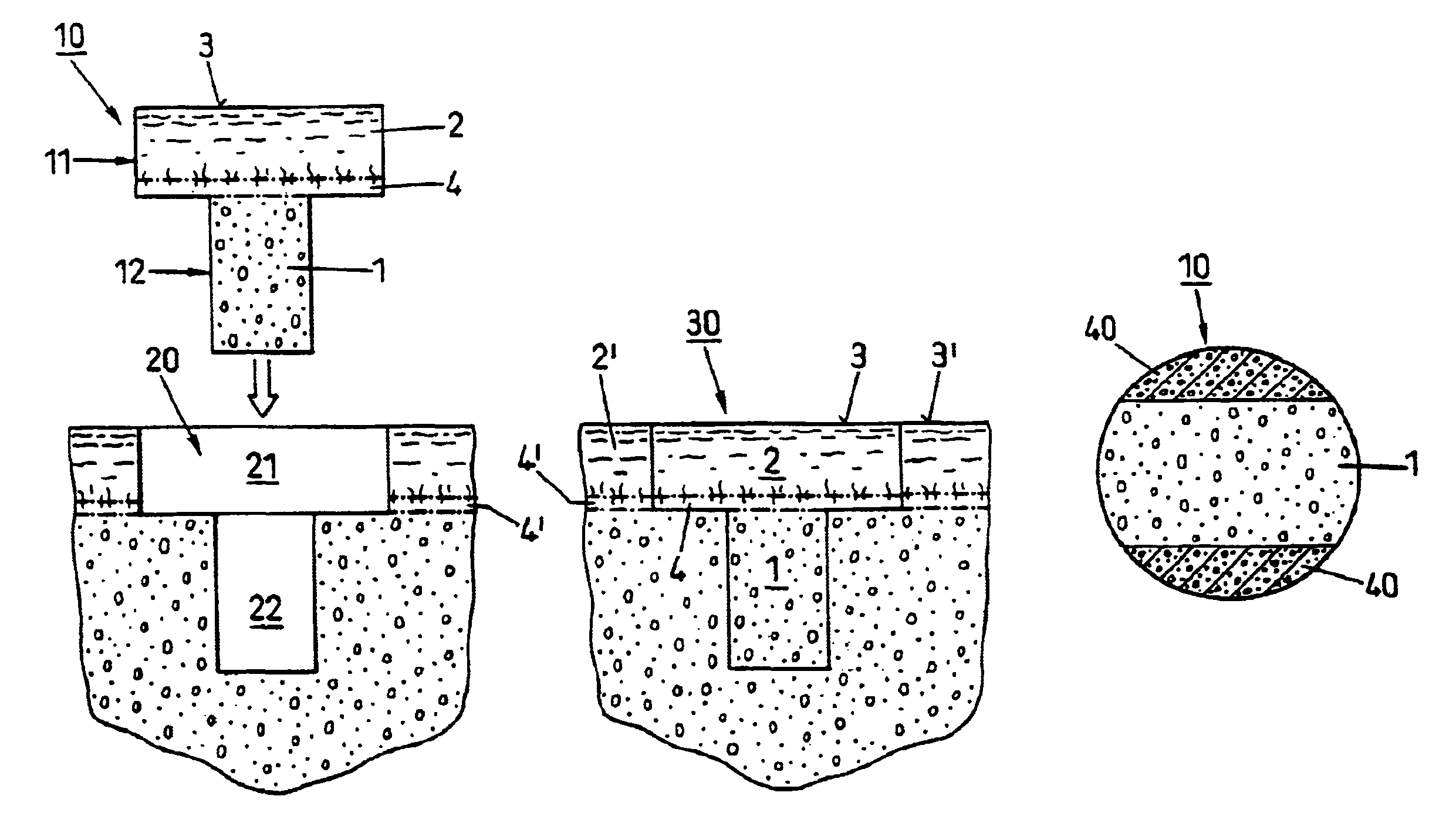
Preparation for repairing cartilage defects or cartilage/bone defects in human or animal joints
a technology for cartilage defects and human or animal joints, applied in the field of medical technology, can solve the problems of articular cartilage damage, very little vascularization, and damage to the bone tissue lying below the articular cartilage, and achieve the effect of reducing resorbability and being resistant to resorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
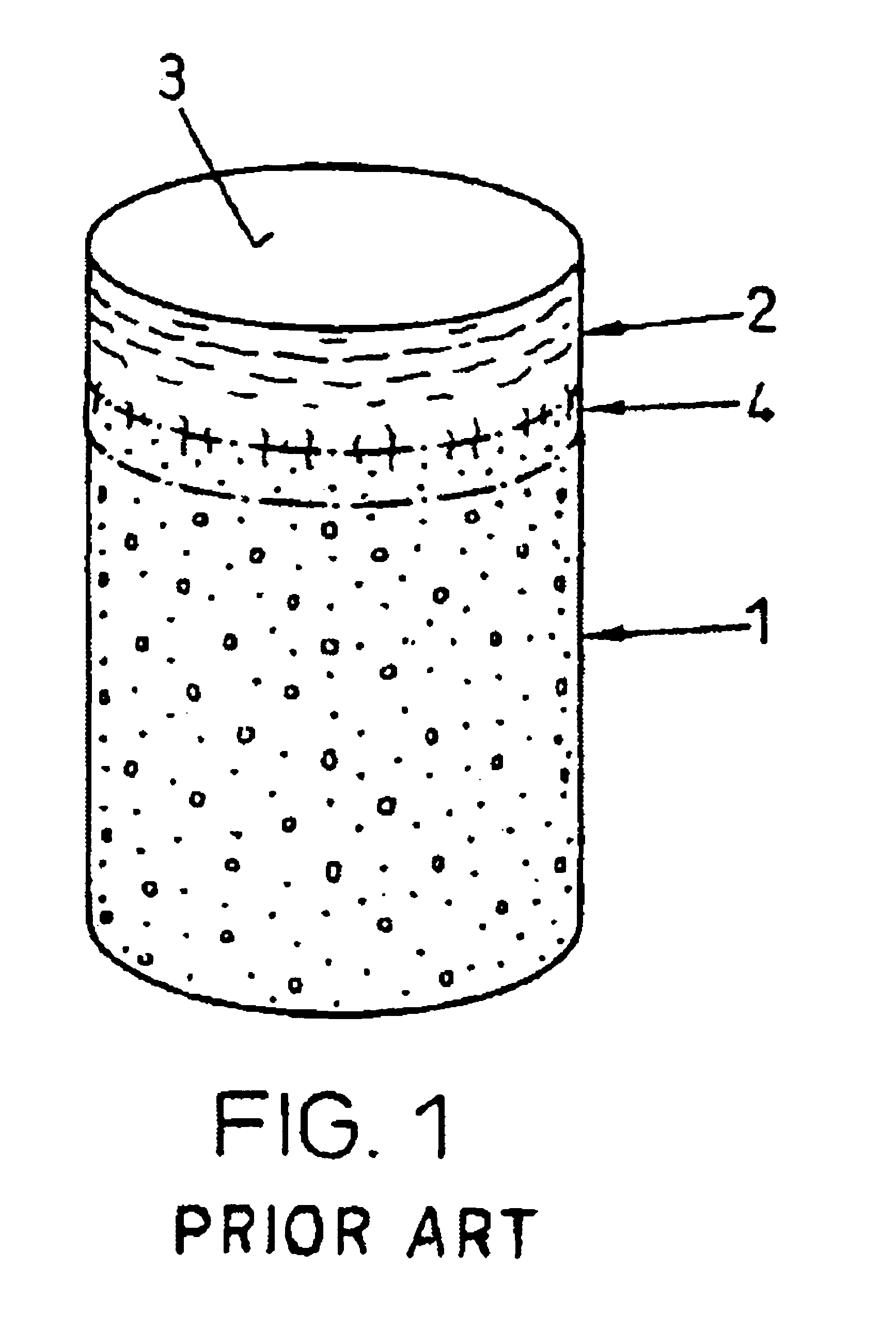
[0034]FIG. 1 shows a device as used according to the state of the art for repairing cartilage defects or cartilage / bone defects in human or animal joints. The device is cylindrical, advantageously with a circular cross section, and comprises a bone part 1 and cartilage layer 2 grown on one end face onto the bone part 1. The cartilage layer 2 forms a cartilage surface 3. Between the bone part 1 and the cartilage layer 2 there extends a subchondral bone plate 4. The transitions from the bone part 1 to the subchondral bone plate 4 and from the subchondral bone plate 4 to the cartilage layer 2 are not visible as lines, as is shown in FIG. 1 in a simplified manner, but they are natural, rather continuous transitions.
[0035]As already mentioned a device as shown in FIG. 1, is harvested from advantageously vital tissue using a hollow drill and is implanted if possible immediately after harvesting (auto-transplants and homo-transplants), or it is removed from joints of slaughtered animals (e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| lengths | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com