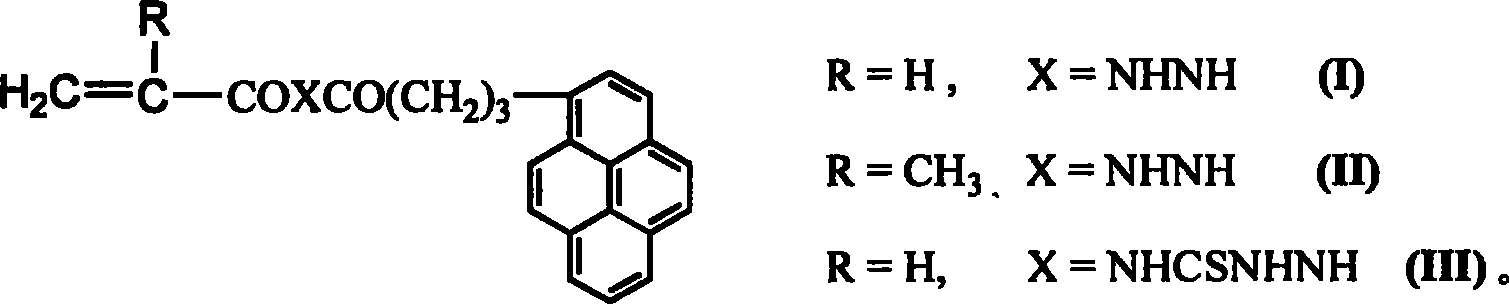Polymerisable fluorescent functional monomer and its prepn and use
A technology of functional monomers and synthesis methods, which is applied in the field of preparation of fluorescent functional monomers, can solve problems such as limitations, difficulty in additives, repeatability, and carcinogenesis-inducing effects, and achieve the effects of simple synthesis methods, easy-to-obtain raw materials, and reasonable synthesis routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Pyrenidine hydrazide and acryloyl chloride in dimethylformamide (DMF) solvent (the molar ratio of pyrene hydrazide to acryloyl chloride is 1:1.2) was stirred at -3°C for 12 hours, and the reaction was carried out by DMF-EtOH-H 2 The functional monomer (I) was obtained by recrystallization from O mixed solvent with a yield of 58.3%. Product structure by MS, 1 H NMR identification: FAB-MS m / z (RI): 356 (38, M + ), 271(58, M-CH 2 CHCONHNH), 215 (43, M-CH 2 CHCONHNHCOCH 2 CH 2 ). 1 H NMR (DMSO-d 6 , δppm): 2.05(t, 2H, CH 2 CH 2 CO), 2.34 (m, 2H, CH 2 CH 2 CO), 2.91(t, 2H, PyCH 2 ), 5.70-6.29 (m, 3H, CH 2 =CH), 7.91-8.42 (m, 9H, PyH), 9.99, 10.12 (each s, 1H, NHNH).
Embodiment 2
[0015] Pyrenidine hydrazide and acryloyl chloride (the molar ratio of pyrene hydrazide to acryloyl chloride is 1:1.8) in dimethylformamide (DMF) solvent were stirred and reacted for 24 hours at 3°C. 2 O mixed solvent recrystallization obtains functional monomer (I), and the yield is 60.5%. Product structure by MS, 1 H NMR identification: FAB-MS m / z (RI): 356 (38, M + ), 271(58, M-CH 2 CHCONHNH), 215 (43, M-CH 2 CHCONHNHCOCH 2 CH 2 ). 1 H NMR (DMSO-d 6 , δppm): 2.05(t, 2H, CH 2 CH 2 CO), 2.34 (m, 2H, CH 2 CH 2 CO), 2.91(T, 2H, PyCH 2 ), 5.70-6.29 (m, 3H, CH 2 =CH), 7.91-8.42 (m, 9H, PyH), 9.99, 10.12 (each s, 1H, NHNH).
Embodiment 3
[0017] The synthetic method of functional monomer (II): pyrene hydrazine and methacryloyl chloride in a mixed solvent of toluene and dimethylformamide (DMF) At 0°C, the reaction was stirred for 15 hours, and the functional monomer (II) was obtained by column chromatography with a yield of 62.2%. Product structure by MS, 1 H NMR identification: FAB-MS m / z (RI): 371(48, M+1), 271(63, Py(CH 2 ) 3 CO + ), 215(52, PyCH 2 + ); 1 H NMR (CDCl 3 , δppm): 1.89 (s, 3H, CH 3 ), 2.19(t, 2H, CH 2 CH 2 CO), 2.34 (m, 2H, CH 2 CH 2 CO), 3.31(t, 2H, PyCH 2 ), 5.35, 5.76 (eachd, 1H, CH 2 =), 7.8-8.2 (m, 9H, PyH), 8.42, 8.51 (each b, 1H, NHNH).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com