N-(2,4-dinitrophenyl)-rhodamine B hydrazide and preparation method and application thereof
A compound and fluorescent probe technology, applied in the field of rhodamine B derivatives and its preparation, can solve the problems of indistinguishable, easy to form interference, short absorption and emission wavelengths, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
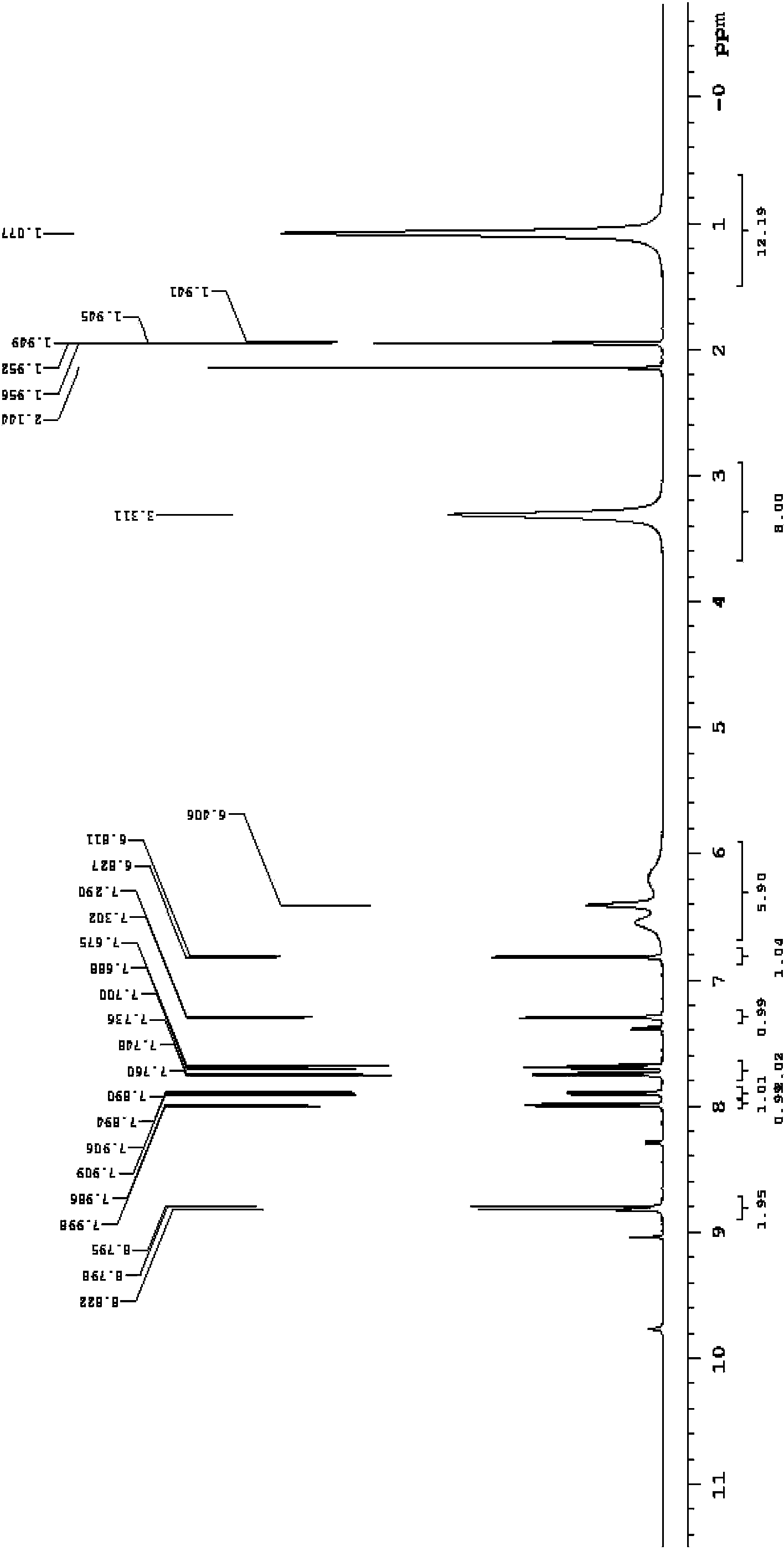
[0031] Example 1. Preparation of optical sensor molecule N-(2,4-dinitrophenyl)-rhodamine B hydrazide (DNPRH)
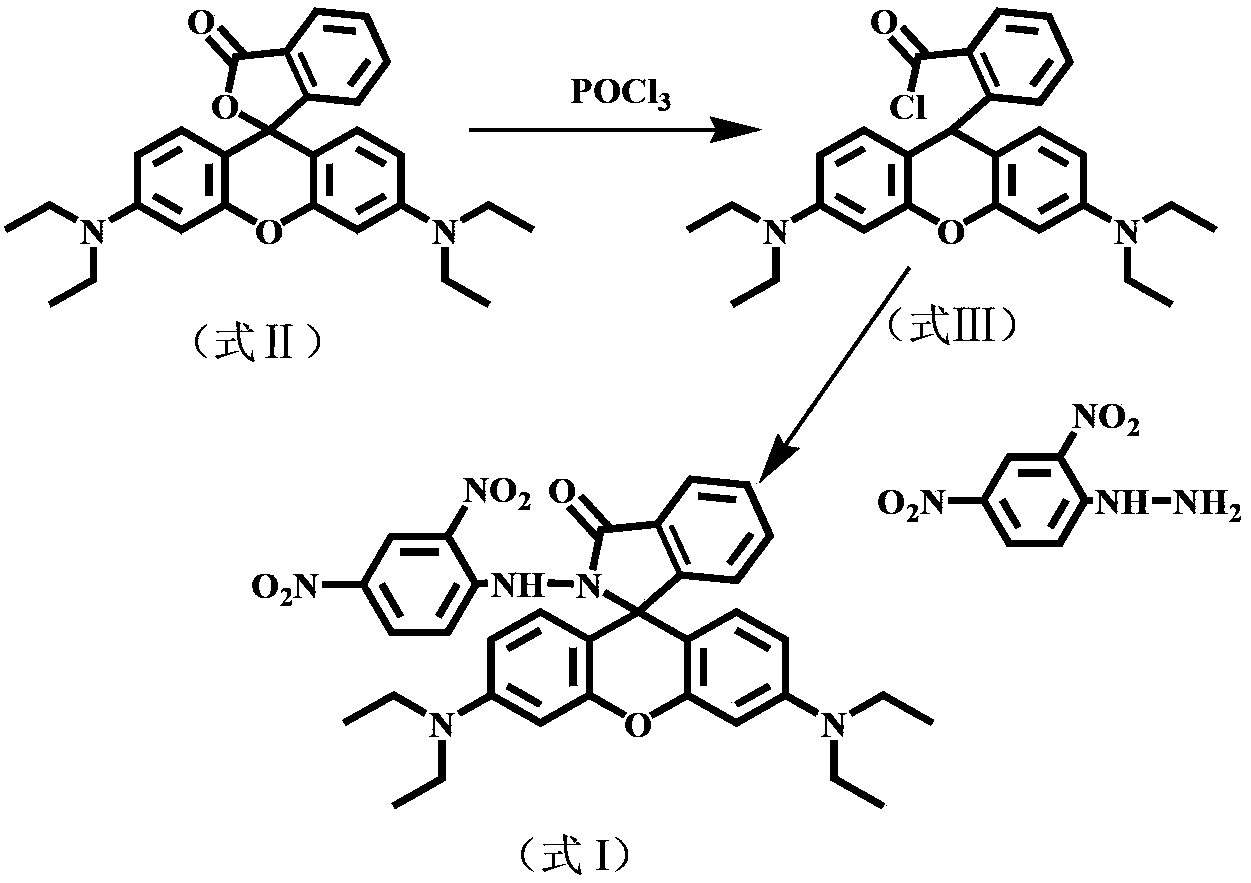
[0032] The reaction process is as figure 1 As shown, the specific method is as follows: Rhodamine B (formula II, 400.0 mg, 0.90 mmol) was dissolved in 10 mL of 1,2-dichloroethane, and 0.8 mL of phosphorus oxychloride was slowly added dropwise to it. The mixed system was heated to reflux for 4 hours under stirring, and then the solvent was evaporated under reduced pressure to obtain rhodamine B acid chloride (formula III) in a purple-red oily state. The crude acid chloride was directly added to 10 mL of acetonitrile to form a solution without purification. Then at room temperature (20-25°C), within 30min, the solution was added dropwise to 30mL 2,4-dinitrophenylhydrazine (0.60g, 3.0mmol) and triethylamine (1.6mL, 11.6mmol ) in acetonitrile solution. Stirring was continued at room temperature (20-25°C) for 6 h, and the solvent was evaporated under reduced pressure to...
Embodiment 2
[0035] Example 2, N-(2,4-dinitrophenyl)-rhodamine B hydrazide (DNPRH) is used as an analytical reagent for Cu 2+ Absorbance detection
[0036] 1. N-(2,4-dinitrophenyl)-rhodamine B hydrazide on Cu 2+ Sensitivity for absorbance detection
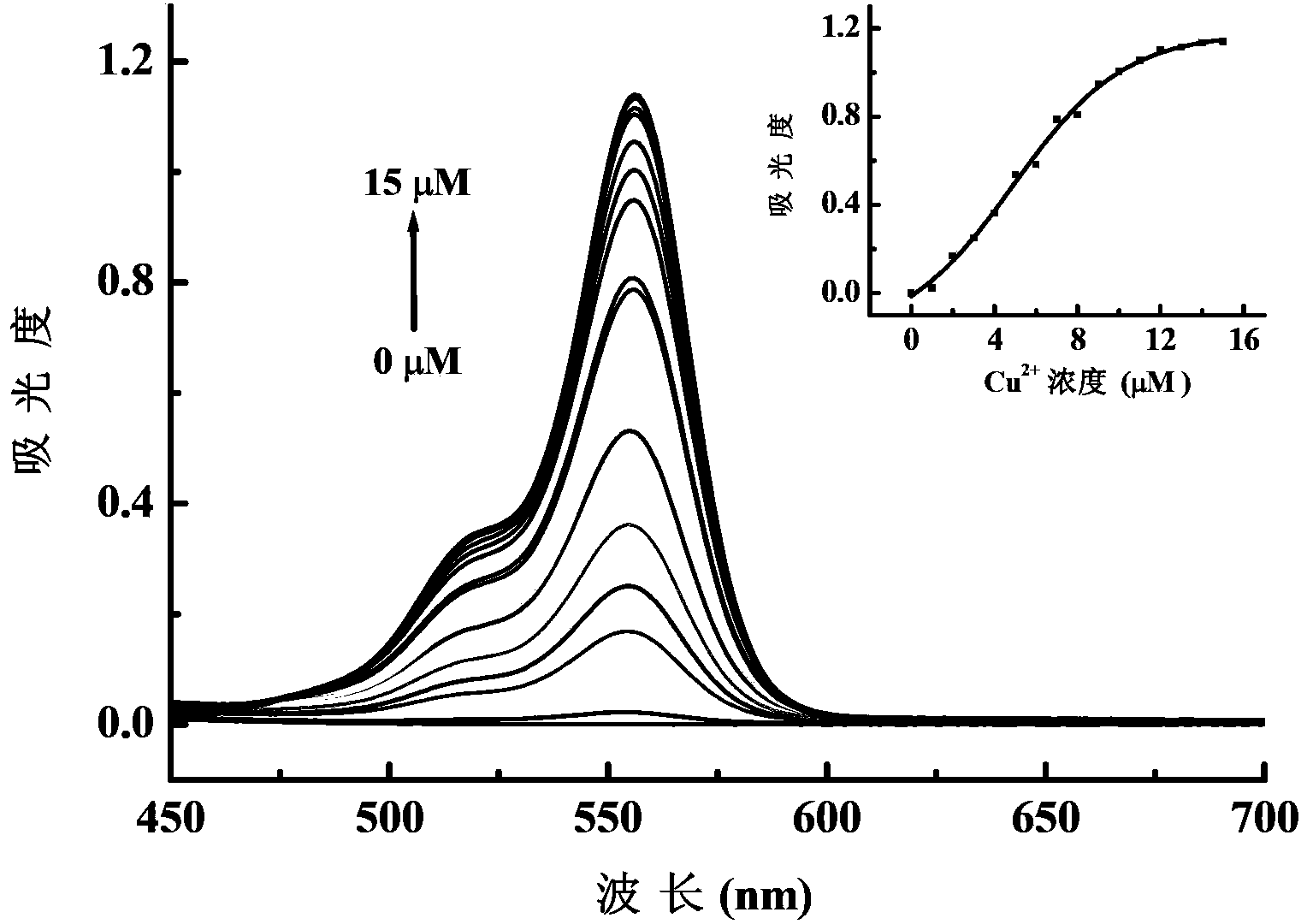
[0037] Add 500 μL of ethanol solution of reagent-N-(2,4-dinitrophenyl)-rhodamine B hydrazide (concentration 1mM) into a 50mL volumetric flask, dilute to 50mL with ethanol, and use it as a dilution of the mother liquor ( Concentration 10 μM). Take 3mL of the above mother liquor dilution respectively, and add an appropriate volume of Cu 2+ stock solution (CuCl 2 solution) (concentration 1mM), so that Cu in the system 2+ Concentrations were 1 ~ 15μM (concentration gradient of 1μM). After reacting at room temperature (20-25°C) for 40min, transfer 3mL of the reaction solution to a 1cm quartz cuvette, use ethanol as a blank, and measure the ultraviolet absorption spectrum. image 3 for Cu 2+ The relationship between the concentration and the...
Embodiment 3
[0040] Example 3, N-(2,4-dinitrophenyl)-rhodamine B hydrazide (DNPRH) is used as an analytical reagent for Cu 2+ Perform fluorescence detection
[0041] 1. N-(2,4-dinitrophenyl)-rhodamine B hydrazide on Cu 2+ Sensitivity for fluorescence detection
[0042] Add 500 μL of ethanol solution of reagent-N-(2,4-dinitrophenyl)-rhodamine B hydrazide (concentration 1mM) into a 50mL volumetric flask, dilute to 50mL with ethanol, and use it as a dilution of the mother liquor ( Concentration 10 μM). Take 3mL of the above mother liquor dilution respectively, and add an appropriate volume of Cu 2+ stock solution (CuCl 2 solution) (concentration 1mM), so that Cu in the system 2+ Concentrations were 1 ~ 15μM (concentration gradient of 1μM). Measure the fluorescence spectrum of the reaction system after reacting at room temperature (20-25°C) for 40 minutes.
[0043] Figure 5 for Cu 2+ The relationship between the concentration and the fluorescence spectrum of the reaction system (the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorption wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com