Screening methods
A cell and electrophysiological technology, applied in the field of screening, which can solve the problems of not being fast, high throughput, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067]Example 1 - Description of the Preferred Embodiment
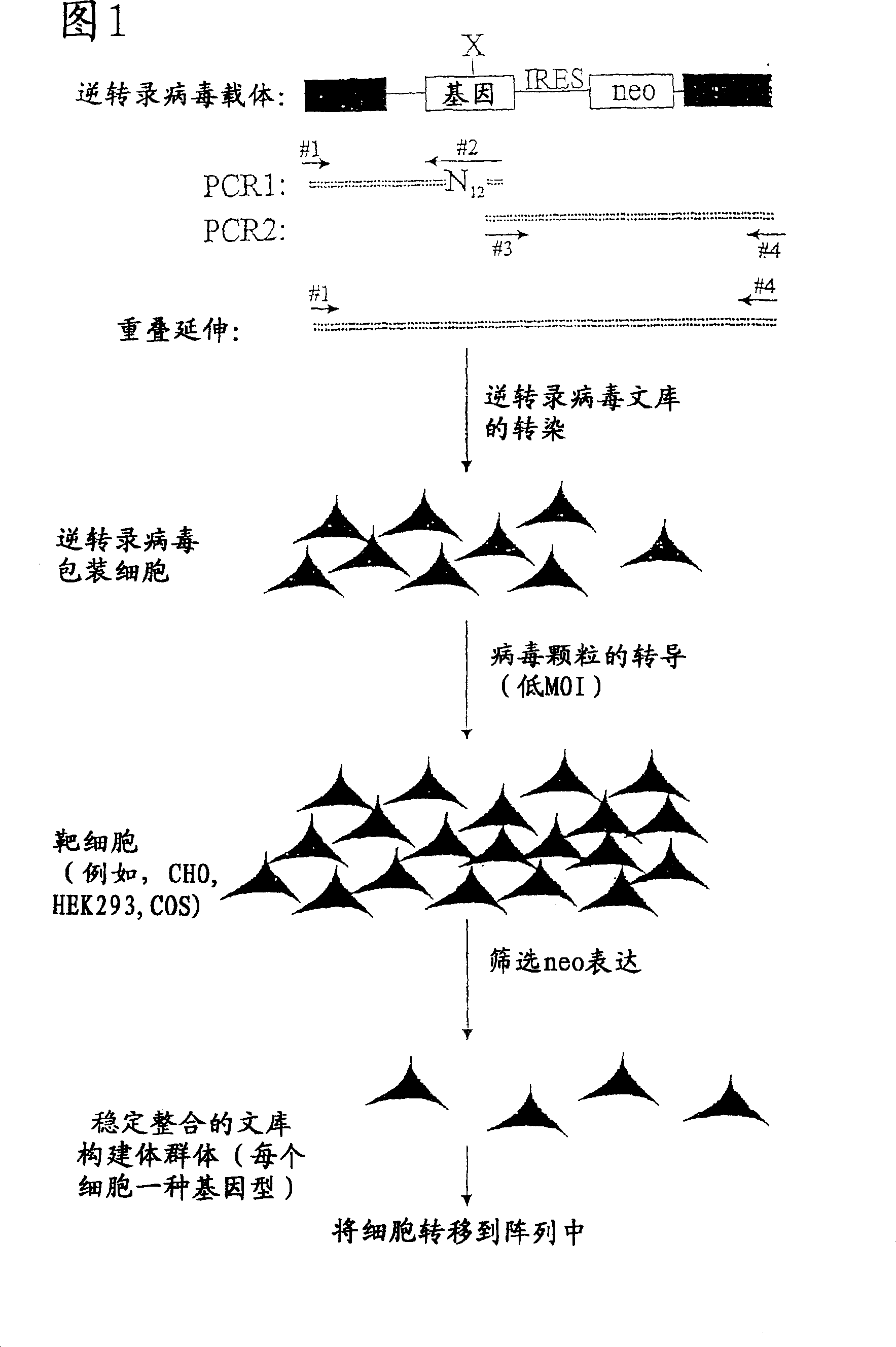
[0068] Prophetic Example: Construction of Genomic Libraries Expressed in Cell Lines
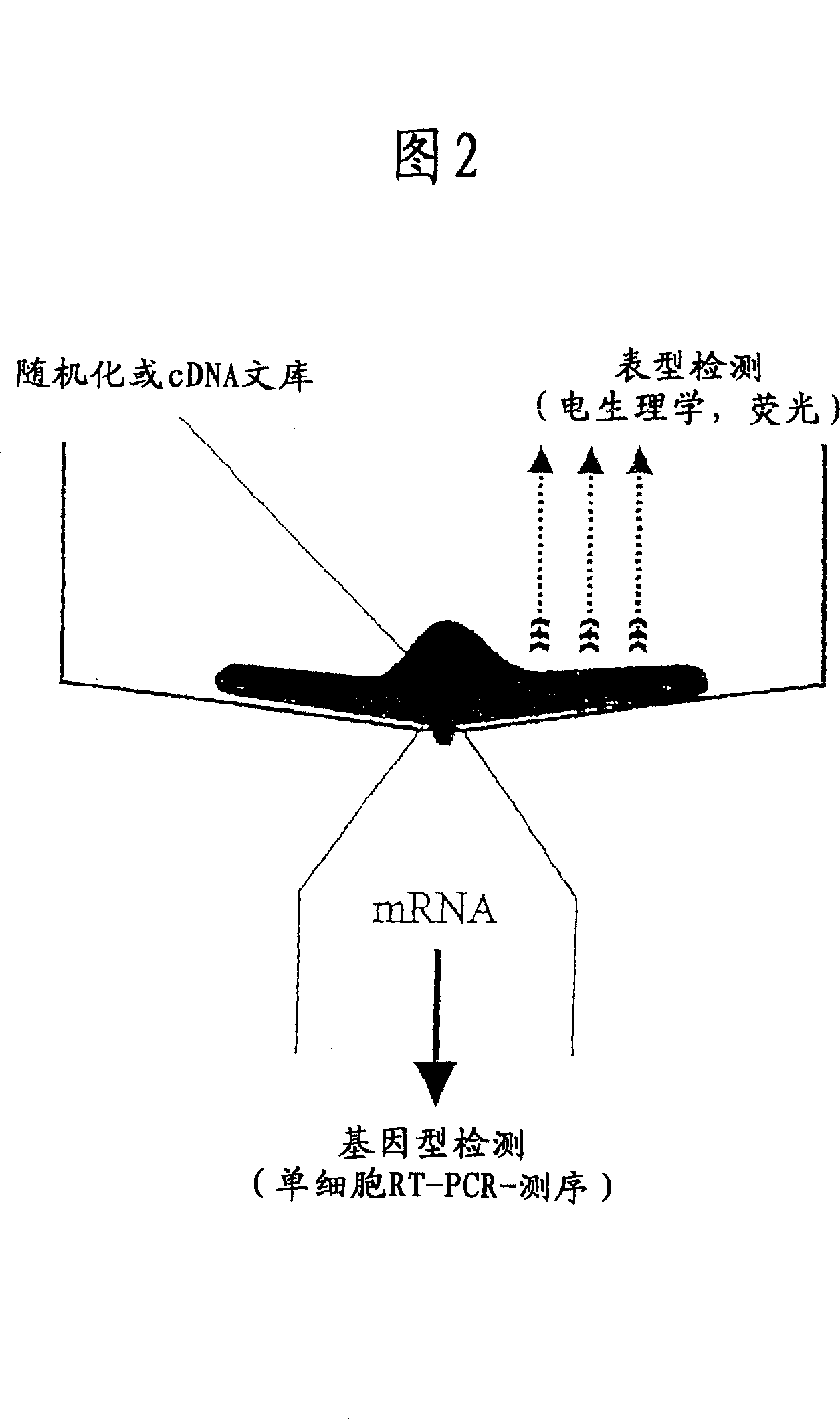
[0069] The purpose of this experiment can be to enhance the binding of a specific ligand to a specific ion channel. This is accomplished by randomizing specific amino acids within ion channels to which ligands are known to bind, including introducing these randomized gene products into cells, and measuring it in a microarray system. Examples of microarray assays are described in Examples 3, 4, and 5.
[0070] The ion channel gene is inserted into a bicistronic retroviral vector that includes all retroviral cis factors necessary to transduce target cells, as well as an internal ribosome entry site from EMCV, followed by neomycin Selected genes (Morgan RA, Couture L, Elroy-Stein O, Ragheb J, Moss B, Anderson WF. Retroviral vectors including a putative internal ribosomal entry site: development of a polycistronic gene transfer system ...
Embodiment 2
[0072] Example 2 - chip for phenotypic analysis
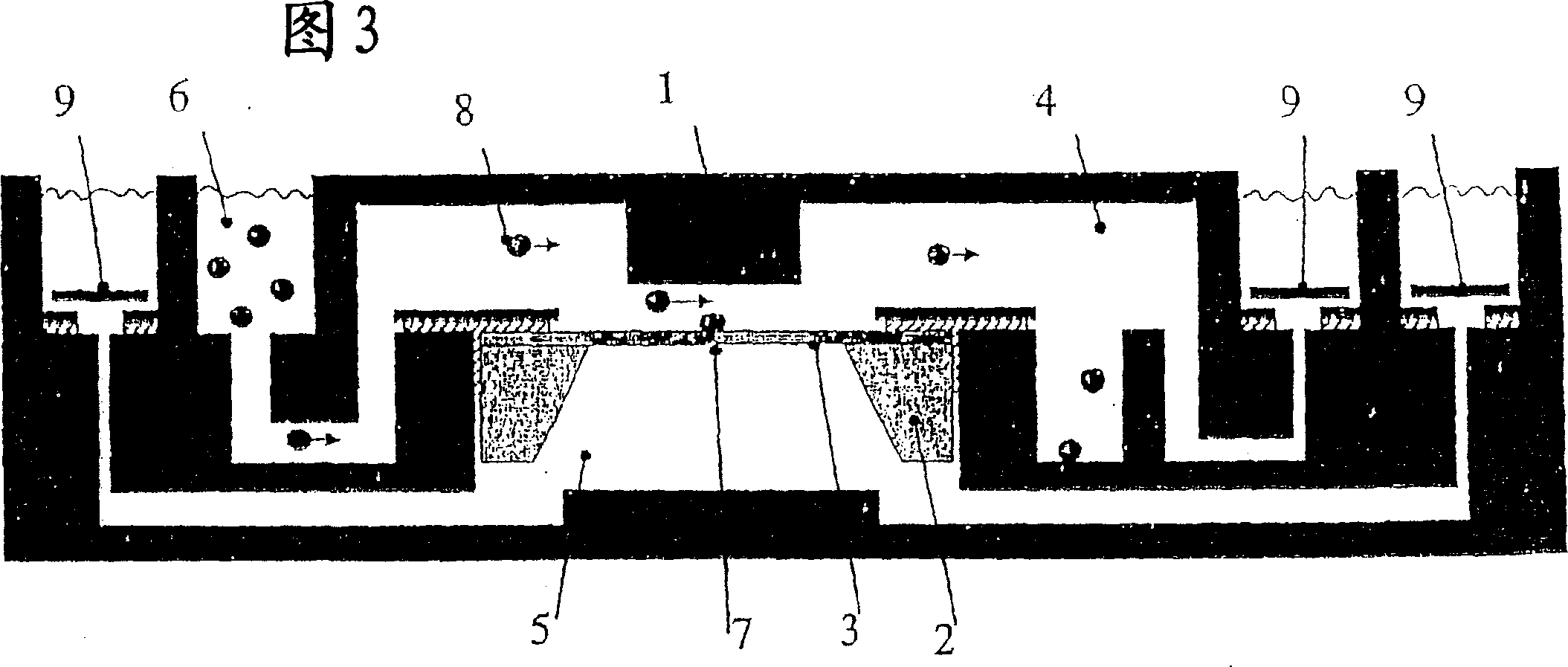
[0073] A suitable chip for patch clamp experiments is disclosed in WO 02 / 9402. For use in the present invention, the chip has been modified so as to be able to extract mRNA from cells, and its structure is shown in FIG. 3 .
[0074] The chip includes a planar substrate including a first surface portion and an opposing second surface portion. The first surface portion has a plurality of sites, each of which is adapted to host a structure including an ion channel.
[0075] Each site includes a measurement electrode associated with it, the substrate carries one or more reference electrodes, the measurement electrodes and the corresponding reference electrodes are brought into electrolytic contact with each other and a potential difference is applied between them An electrode capable of generating a current between them by transporting ions from one electrode and receiving ions from the other.
[0076] Each of said sites is adap...
Embodiment 3
[0083] Example 3 - Electrophysiological Assays
[0084] Electrophysiological assays can be performed with chip constructs made from the matrix and used as described above and shown in Figure 3 to perform patch clamp tests by applying voltage changes to the test cells.
[0085] Typical assays involve the addition of specific test samples to assay devices, for which each assay device is isolated from the other assay devices to avoid mixing of test samples and conduction of electrical current between the devices. In addition, assays are performed on control cells that have not been transformed with the heterologous DNA sequence in order to provide control phenotypic parameters for the cell line.
[0086] Multiple test cells can be studied at any time and the electrophysiological assays and analyzes performed to confirm which test cells exhibit a different phenotype, if any, from the control cells.
[0087] Electrophysiological measurements generated by applying voltage changes t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com