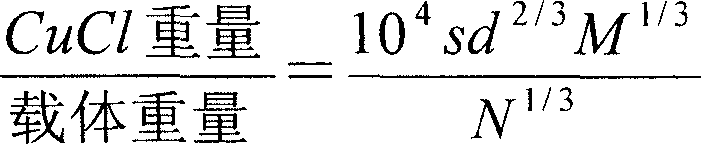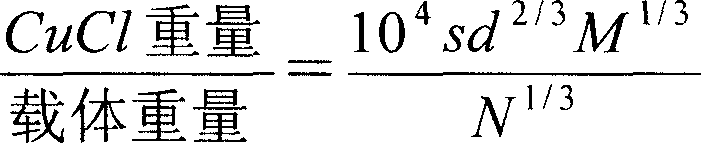Method of separating alpha ethyl linolenate by cuprous chloride complexation adsorption
A technology of complexation and adsorption of ethyl linolenate, applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylic acid esters, etc., can solve problems such as loss of complexation, high price, and poor separation effect of fatty acids. Achieve good separation effect and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Take Al with a specific surface area of 127.85m2 / g 2 o 3 50g, the molecular weight of CuCl is 99, the density is 4.14, by (s is the specific surface of the carrier, M is the molecular weight of CuCl, d is the density of CuCl, and N is the Avogadro constant) the formula calculation shows that CuCl9g, 9g CuCl and 50g Al 2 o 3 Mix well, and calcined at 350°C for 4h in a muffle furnace. After cooling to room temperature, weigh 50 g and load it into a 19 mm×3 cm adsorption column by wet method.
[0020] Mix linseed oil and ethanol at a ratio of 1:3 (molar ratio), and when the temperature reaches 50° C., add lipase accounting for 5% of the total weight of the reactants, and start stirring for 8 hours to obtain mixed fatty acid ethyl esters.
[0021] Dissolve 1ml of mixed fatty acid ethyl ester in 10ml of petroleum ether, add to the adsorption column, then add 90ml of petroleum ether to start elution, collect 100ml of fractions, add 100ml of petroleum containing aceton...
Embodiment 2
[0023] Mix 9g CuCl with 50g Al 2 o 3 Mix well, and calcined at 380°C for 2h in a muffle furnace. After cooling to room temperature, 50 g was weighed and packed into a column (19 mm×3 cm) by wet method.
[0024] Mix perilla oil and ethanol at a ratio of 1:3 (molar ratio), and when the temperature reaches 50° C., add lipase accounting for 5% of the total weight of the reactants, and start stirring for 8 hours to obtain mixed fatty acid ethyl esters. Its fatty acid composition is shown in Table 1.
[0025] Dissolve 1ml of mixed fatty acid ethyl ester in 10ml of n-hexane, add to the adsorption column, then add 90ml of n-hexane to start elution, collect 100ml of fractions, then add 100ml of n-hexane containing 1%, 2%, and 4% acetone Alkanes continued to elute until the sample completely flowed out of the adsorption column. One fraction was collected per 100ml, and a total of 4 fractions were collected, which were recorded as 1#, 2#, 3#, and 4#. No α-linolenic acid ethyl ester ...
Embodiment 3
[0029] Mix 9g CuCl with 50g Al 2 o 3 Mix well, and calcined at 350°C for 4h in a muffle furnace. After cooling to room temperature, weigh 50 g and load it into a 19 mm×3 cm adsorption column by wet method.
[0030] Dissolve 1ml of mixed fatty acid ethyl ester in 10ml of petroleum ether n-hexane mixed solvent (V:V=50:50), add to the adsorption column, then add 90ml of the above mixed solvent to start elution, and collect 100ml of fraction Then add 100ml of acetone-containing 1%, 2%, and 4% of the above-mentioned mixed solvents to continue eluting until the sample completely flows out of the adsorption column. No α-linolenic acid ethyl ester was detected in 1# fraction, α-linolenic acid ethyl ester accounted for 41.23% of all fatty acid ethyl esters in 2# fraction, α-linolenic acid ethyl ester accounted for all fatty acid ethyl esters in 3# fraction 76.25% of esters. The ethyl α-linolenic acid in fraction 4# accounted for 93.27% of all fatty acid ethyl esters.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com