Ring-opening polymerization catalyst for lactide and process for preparing same
A ring-opening polymerization and catalyst technology, applied in the field of lactide ring-opening polymerization catalyst and its preparation, can solve the problems of high temperature, low activity, long time, etc., and achieve the effect of rapid stereoselective synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
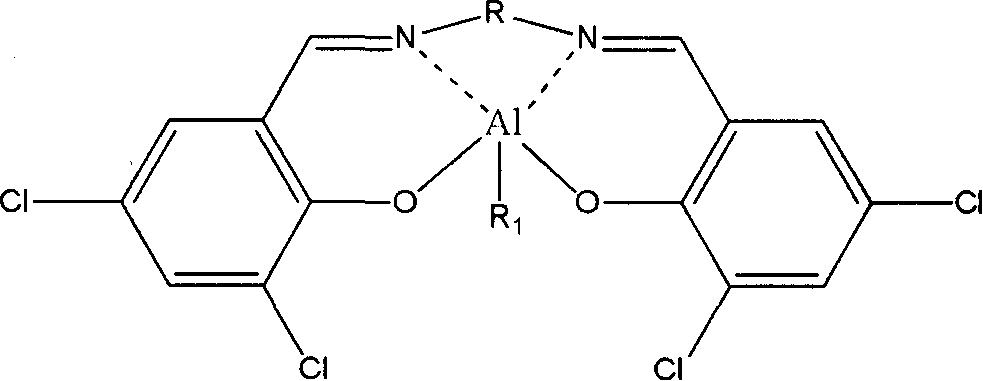
[0044] Synthesis and purification of 3,5-dichlorosalicylaldehyde ethylenediamineethylaluminum (Cat1a)
[0045] Dissolve 2.0 g of ethylenediamine in 5 ml of absolute ethanol, and 12.7 g of 3,5-dichlorosalicylaldehyde in 190 ml of absolute ethanol, wherein the molar ratio of ethylenediamine 3,5-dichlorosalicylaldehyde is 1:2. Slowly drop this ethylenediamine absolute ethanol solution into the stirred 3,5-dichlorosalicylaldehyde absolute ethanol solution at 80°C through a constant pressure funnel, and react for 0.5 hours to obtain 11.2 g of 3,5 -Dichlorosalicylaldehyde ethylenediamine ligand. 3g of 3,5-dichlorosalicylaldehyde ethylenediamine ligand was redissolved in 300ml of cyclohexane, heated to 100°C to dissolve completely, and then slowly lowered to room temperature to obtain 3,5-dichlorosalicylaldehyde ethyl Diamine ligand crystals.
[0046] Using anhydrous and oxygen-free technology, under the protection of argon, 1.0 g of purified 3,5-dichlorosalicylaldehyde ethylenedia...
Embodiment 2
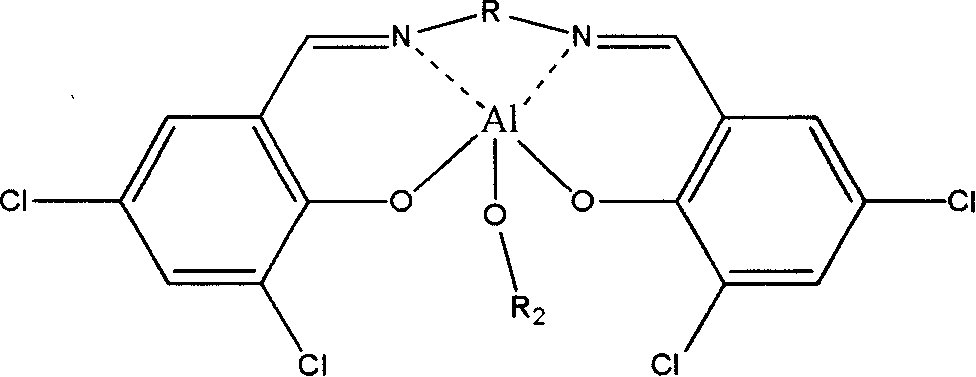
[0048] Synthesis and Purification of 3,5-Dichlorosalicylaldehyde Ethylenediamine Isopropoxy Aluminum Catalyst (Cat1b)
[0049] Using anhydrous and oxygen-free technology, under argon protection, 1.0 g of purified 3,5-dichlorosalicylaldehyde ethylenediamine ethylaluminum catalyst was dissolved in 5 ml of anhydrous toluene solvent, and 0.13 g of isopropanol was extracted. Add 2.6 ml of anhydrous toluene solvent to the stirred anhydrous toluene solution of 3,5-dichlorosalicylaldehyde ethylenediamine ethyl aluminum catalyst, heat up and reflux for reaction, and react at 80°C for 72 hours, then slowly lower the temperature to At room temperature, Cat1b was obtained.
Embodiment 3
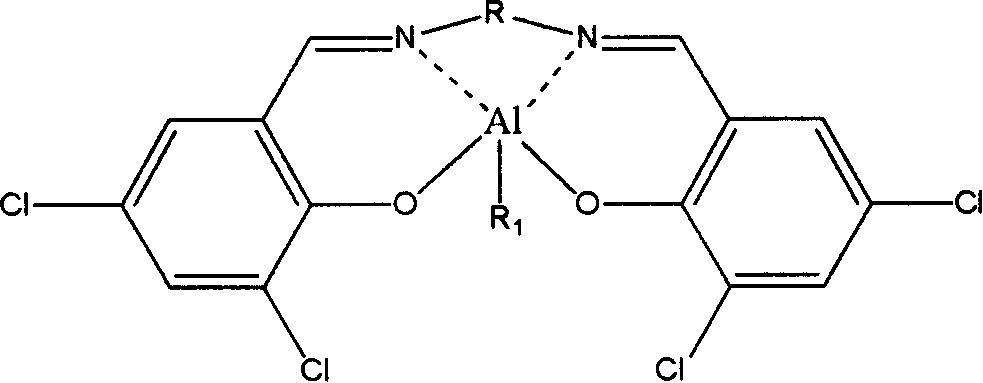
[0051] Synthesis and Purification of 3,5-Dichlorosalicylaldehyde Propylenediamine Methylaluminum Catalyst
[0052] Dissolve 2.5g of propylenediamine in 50ml of absolute ethanol, and dissolve 14.1g of 3,5-dichlorosalicylaldehyde in 1400ml of absolute ethanol, wherein the molar ratio of propylenediamine to 3,5-dichlorosalicylaldehyde is 1:2.2 . The absolute ethanol solution of propylenediamine was slowly added dropwise to the stirred 3,5-dichlorosalicylaldehyde absolute ethanol solution at 100°C through a constant pressure funnel, and reacted for 14 hours to obtain 14.9 g of 3,5- Dichlorosalicylaldehyde propylenediamine ligand. Redissolve 3g of 3,5-dichlorosalicylaldehyde propylenediamine ligand in 450ml of cyclohexane, raise the temperature to 85°C to dissolve it completely, and then slowly lower it to room temperature to obtain the purified 3,5 -Dichlorosalicylaldehyde propylenediamine ligand crystal.
[0053] Using anhydrous and oxygen-free technology, under argon protecti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| dispersity | aaaaa | aaaaa |
| dispersity | aaaaa | aaaaa |
| dispersity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com