Remedy for corneal failure
A technology of cornea and corneal sensitivity, applied in sensory diseases, nervous system diseases, peptide/protein components, etc., can solve problems such as not found
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
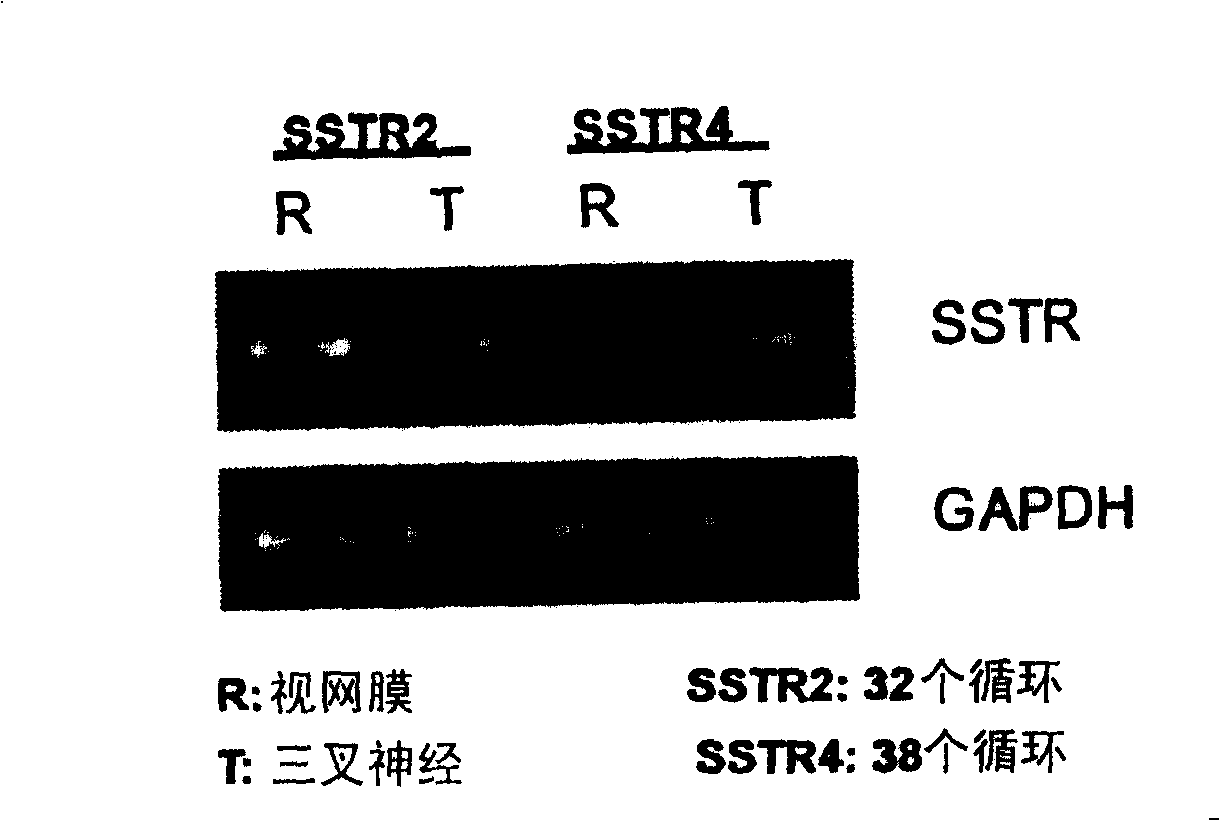
[0124] Experimental Example 1 Expression of Somatostatin Receptor in Rabbit Trigeminal Nerve
[0125] 1) Animals used
[0126] Japanese white rabbits (body weight 2.0 kg) purchased from Fukusaki Rabbit Warren were used.
[0127] 2) Test method
[0128] Celactal (xylazine): ketamine injection (ketamine hydrochloride) = 0.5: 1 intramuscular injection (0.9 mL / kg) was given to Japanese white rabbits for general anesthesia. After cardiac perfusion with saline, the retina and trigeminal nerve were harvested separately. RNA was extracted from each tissue by the AGPC method using TRIzol reagent (manufactured by GIBCO BRL), genomic DNA was removed by DNase treatment, and a reverse transcription reaction was performed using SuperScript II (manufactured by GIBCO BRL). The cDNA was amplified under the reaction conditions described in Table 1 using Platinum Taq DNA polymerase (GIBCO BRL). As primers, the rabbit somatostatin receptor SSTR2 gene-specific primer (hereinafter referred to a...
experiment example 2
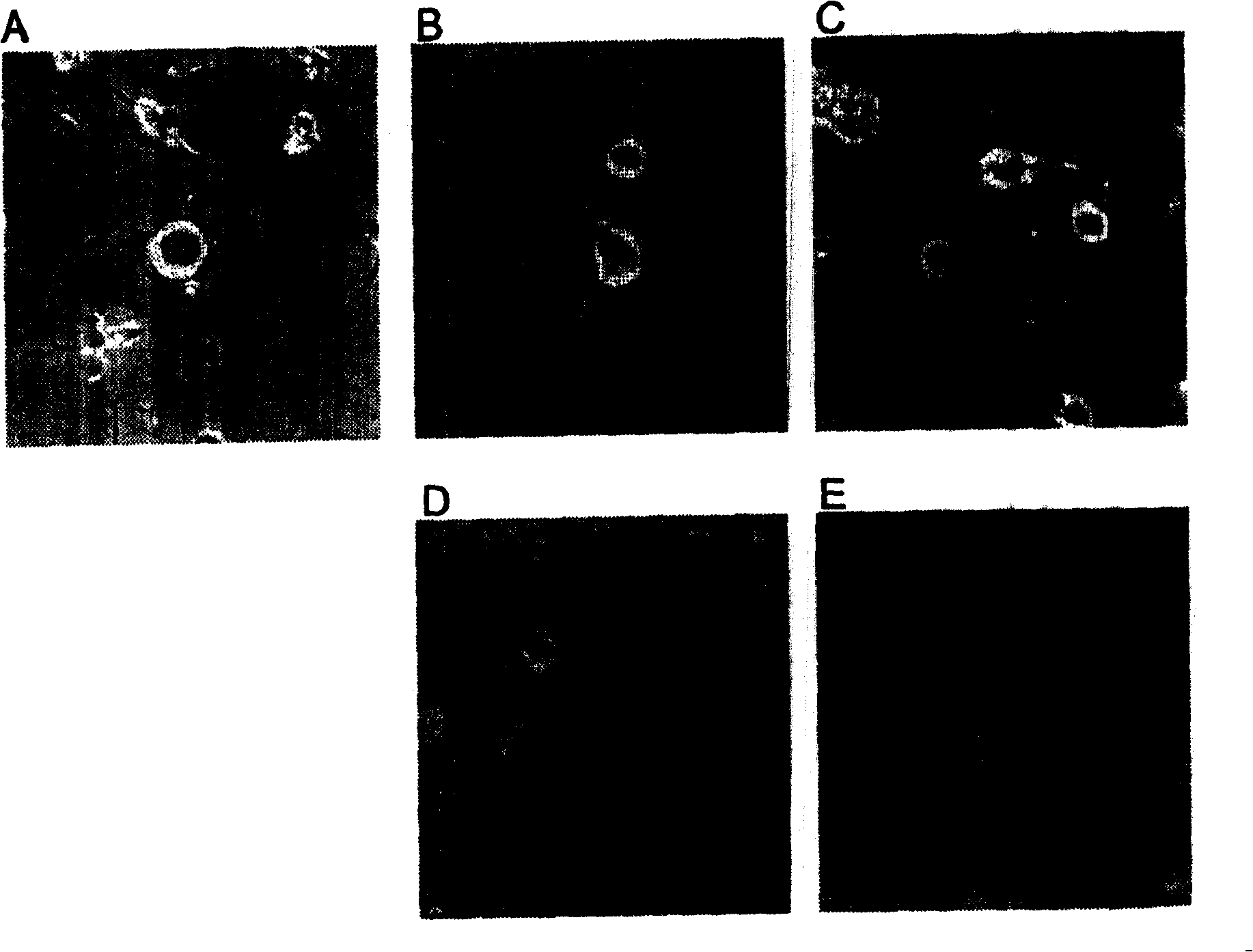
[0134]Axon extension promotion in cultured rabbit trigeminal nerve cells (in vitro experiment)
[0135] 1) Animals used
[0136] Japanese white rabbits (2-3 days old) purchased from Fukusaki Rabbit Warren were used.
[0137] 2) Test substance
[0138] As test substances, somatostatin (manufactured by CALBIOCHEM, Lot B33795) and NGF (NGF-7S, manufactured by Sigma) were used. Test substances were dissolved in phosphate buffered saline (PBS) to 100 μM somatostatin, 20 μg / mL NGF-7S. Prepared reagents were stored at -80°C and dissolved before use.
[0139] 3) Test method
[0140] Trigeminal neurons were isolated as reported by Chan et al. (Kuan Y. Chan and Richard H. Haschke. Exp. Eye Res. 41:687-699, 1985). More specifically, after the rabbit heart was perfused with saline under ether anesthesia, the trigeminal ganglion was excised, and the trigeminal ganglion was dispersed using a nerve dispersion solution (SUMITOMOBAKELITE Co., Ltd.), and the cells were plated on polymer-co...
experiment example 3
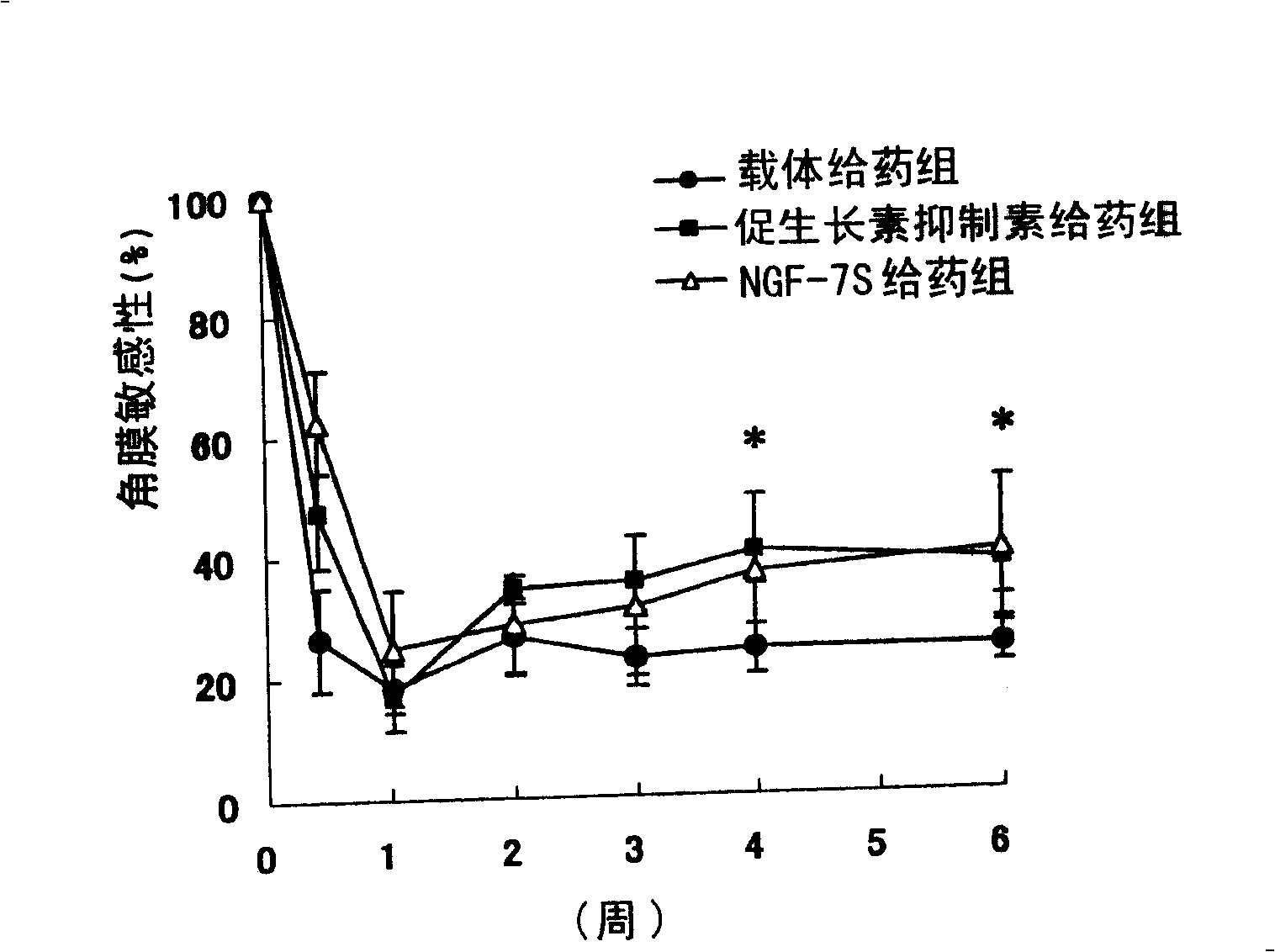
[0152] Functional changes in corneal sensitivity after corneal nerve dissection in rabbits (in vitro assay)
[0153] 1) Animals used
[0154] Male Japanese white rabbits (body weight 1.5kg-2.0kg) purchased from Fukusaki Rabbit Warren were used.
[0155] 2) Test substance
[0156] As test substances, somatostatin (manufactured by CALBIOCHEM, Lot B33795) and NGF (NGF-7S, manufactured by Sigma) were used. The test substance was dissolved in the vehicle shown below and used for the test.
[0157] Carrier formulation
[0159] Sodium dihydrogen phosphate dihydrate 0.1g
[0160] Appropriate amount of sodium hydroxide
[0161] (pH 7.0)
[0162] Appropriate amount of distilled water for injection
[0163]
[0164] Total 100mL
[0165] 3) Test method
[0166] Celactal (xylazine): ketamine injection (ketamine hydrochloride) = 0.5: 1 intramuscular injection (0.9 mL / kg) was given to the r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com