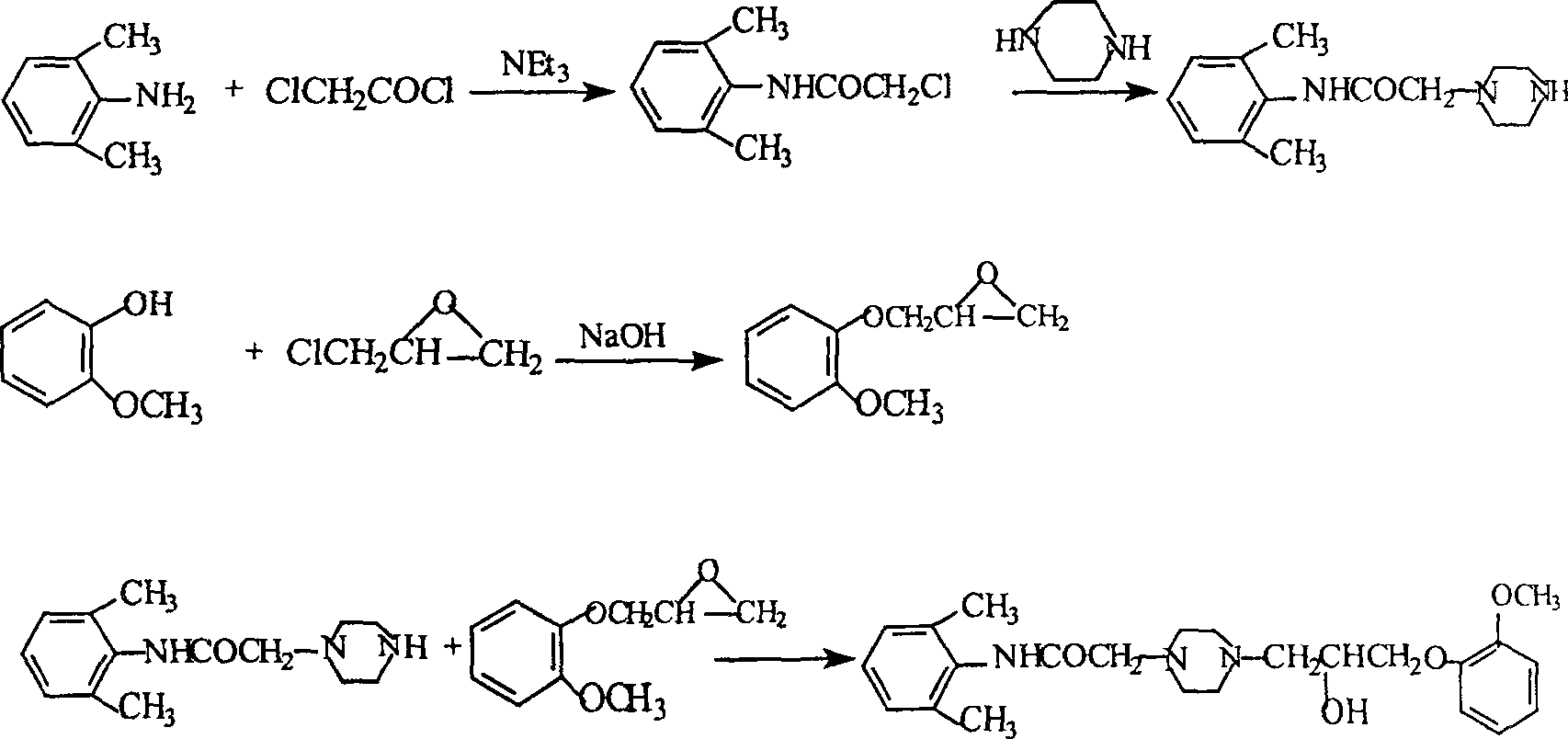
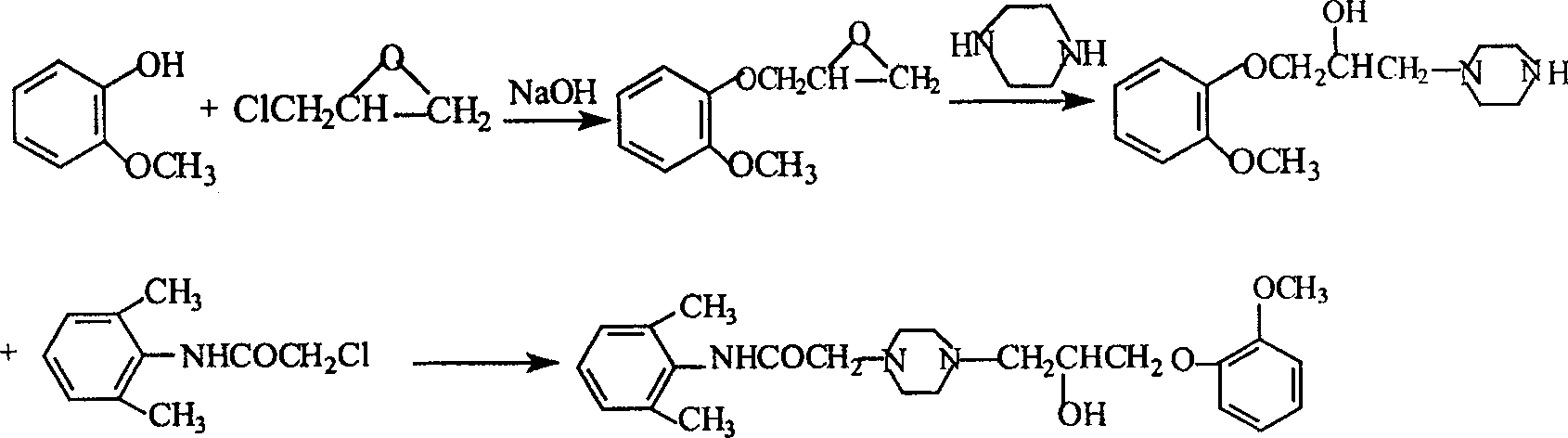
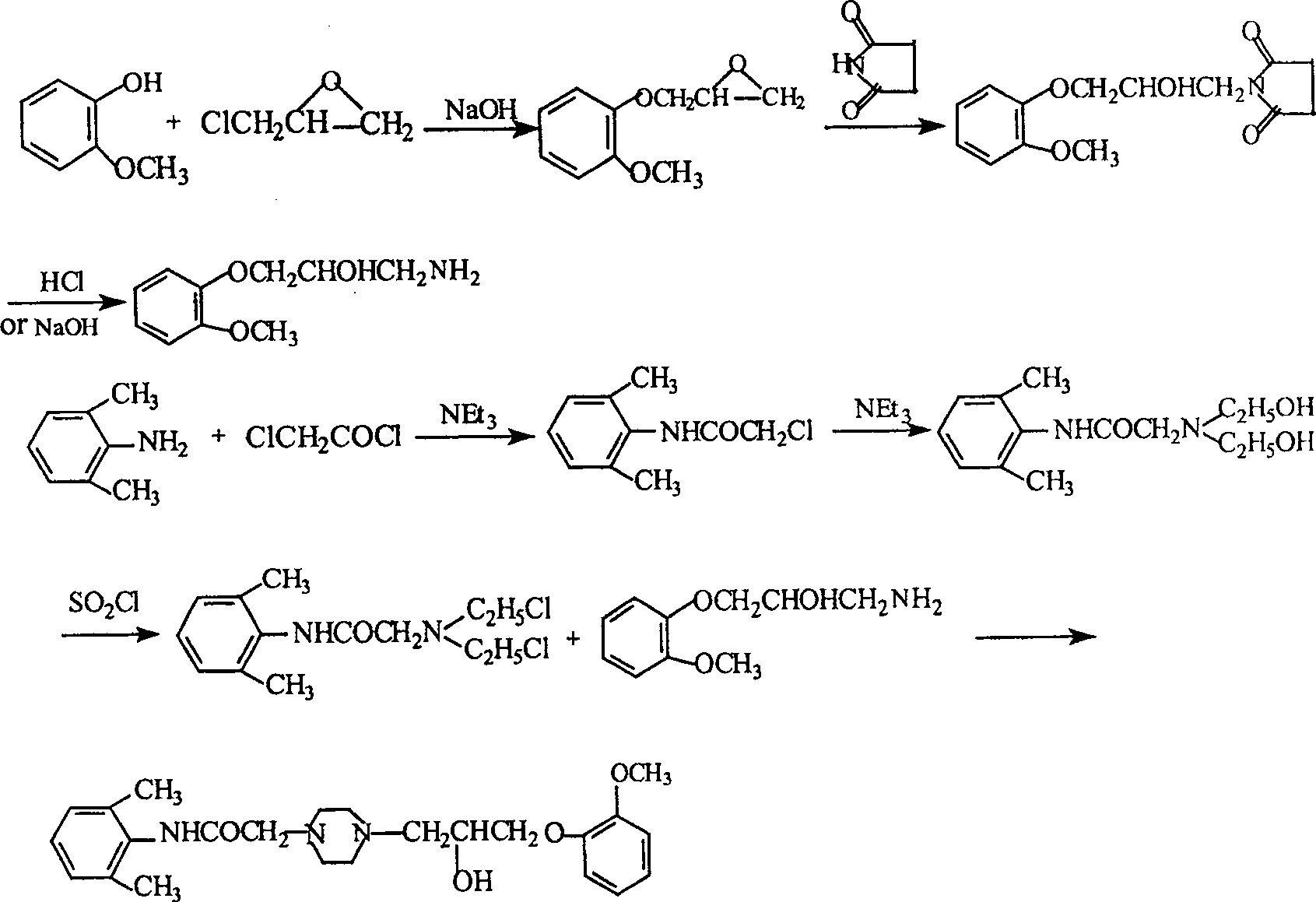
Method for synthesizing Ranolazine
A synthetic method, the technology of ranolazine, which is applied in a new synthetic field, can solve the problems of short reaction steps, long reaction steps, difficulties in the synthesis of ranolazine, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1. Preparation (yield: 80%) of 3-(2-methoxyphenoxy)-1,2-epoxypropane (intermediate I) feed ratio: o-methoxyphenol: epichlorohydrin di Hexane: water: NaOH=1:1.68:2.7:1.2:0.36(w / v / v / w)
[0032] Add 150g of o-methoxyphenol, 378ml of dioxane, 168ml of water and 50g of NaOH into a 2L three-necked reaction flask, add 252ml of epichlorohydrin under stirring at room temperature, and react under reflux for 2 hours. Cool to room temperature, add ethyl acetate, filter, separate the organic layer, extract the aqueous layer twice with ethyl acetate, combine the organic layers, dry with anhydrous sodium sulfate, distill under reduced pressure, collect 121-124 ° C / 2KPa fractions to obtain 163g product.
[0033] 2. Preparation of 2-chloro-N-(2,6xylyl)acetamide (yield: 84.3%)
[0034] Feed ratio: 2,6-dimethylaniline: chloroacetyl chloride: triethylamine: toluene = 1:0.94:1.0:10 (w / w / w / v)
[0035] Add 165g of 2,6-dimethylaniline, 165g of triethylamine, and 1,650ml of toluene into a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com