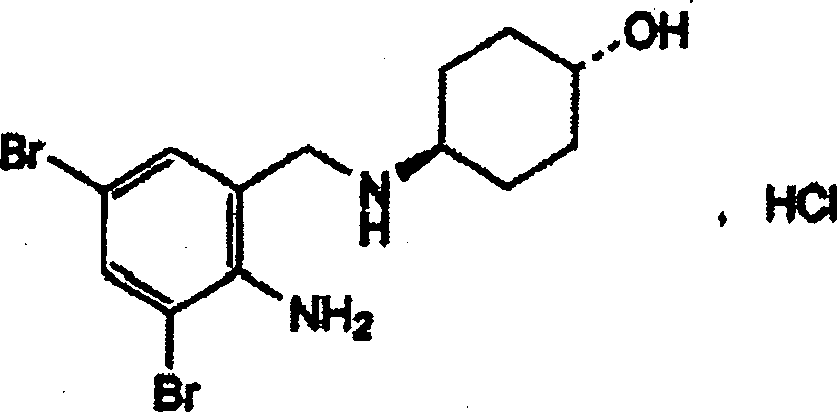
Ambroxol hydrochloride freeze-dried powder injection and preparing method thereof
A technology of ambroxol hydrochloride and freeze-dried powder injection is applied in the field of preparation of ambroxol hydrochloride freeze-dried powder injection and ambroxol hydrochloride freeze-dried powder injection, and can solve problems such as the influence of main drug stability, unknown side effects and the like, Achieve the effect of easy dissolution, avoid interaction, and simple excipients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 Preparation of freeze-dried powder containing 15 mg of ambroxol hydrochloride in each bottle, 1000 bottles in total.
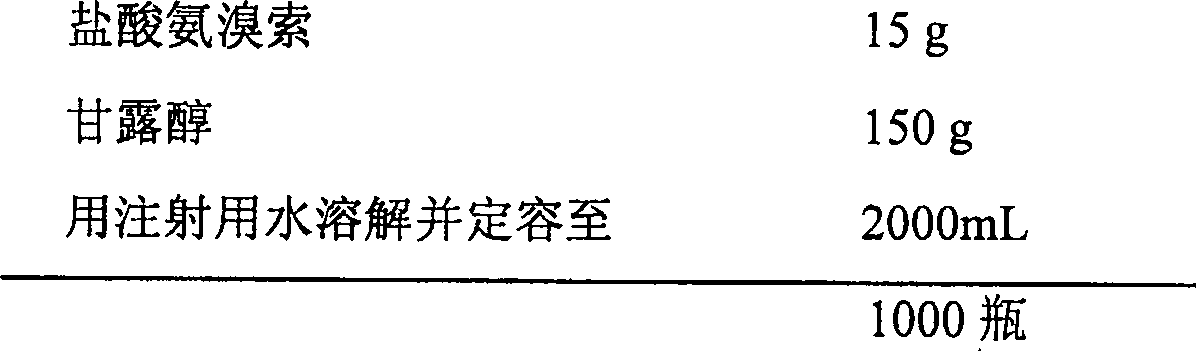
[0046] The composition of the freeze-dried powder containing 15mg of ambroxol hydrochloride in each bottle:
[0047]
[0048] Dissolve 15g of ambroxol hydrochloride in sterile water for injection, stir to dissolve, weigh 100g of mannitol, add to the above solution, stir to dissolve, heat to 60°C, stir to dissolve, and use 0.1mol / L hydrochloric acid solution and 0.1mol / L sodium hydroxide solution to adjust the pH of the solution to 4.9, add sterilized water for injection to 2000mL, add 0.1% (g / mL) activated carbon, stir for 14 minutes, and then filter the liquid through a 0.22um filter membrane. The filtrate was divided into vials, placed in a freeze dryer that had been cooled to -20°C, and kept frozen at -20°C for 3 hours, then lowered to -45°C, vacuumed, and heated to -7°C, the time to maintain -7°C is 6 hours, and then continue to heat u...
Embodiment 2
[0049] Example 2 Preparation of freeze-dried powder containing 15 mg of ambroxol hydrochloride in each bottle, 1000 bottles in total.
[0050] The composition of the freeze-dried powder containing 15mg of ambroxol hydrochloride in each bottle:
[0051]
[0052] Dissolve 15g of ambroxol hydrochloride in sterile water for injection, stir to dissolve, weigh 150g of mannitol, add to the above solution, stir to dissolve, heat to 60°C, stir to dissolve, and use 0.1mol / L hydrochloric acid solution and 0.1mol / L sodium hydroxide solution to adjust the pH of the solution to 5.1, add sterilized water for injection to 2000mL, add 0.1% (g / mL) activated carbon, stir for 12 minutes, and then filter the medicinal solution through a 0.22um filter membrane. The filtrate was divided into vials, placed in a freeze dryer that had been cooled to -18°C, and kept frozen at -18°C for 3 hours, then lowered to -45°C, vacuumed, and heated to -10°C, the time to maintain -10°C is 6 hours, and then cont...
Embodiment 3
[0053] Example 3 Preparation of freeze-dried powder containing 15 mg of ambroxol hydrochloride in each bottle, 1000 bottles in total.
[0054] The composition of the freeze-dried powder containing 15mg of ambroxol hydrochloride in each bottle:
[0055]
[0056]
[0057] Dissolve 15g of ambroxol hydrochloride in sterile water for injection, stir to dissolve, weigh 68g of mannitol and add to the above solution, stir to dissolve, heat to 60°C, stir to dissolve, and use 0.1mol / L hydrochloric acid solution and 0.1mol / L sodium hydroxide solution to adjust the pH of the solution to 4.8, add sterilized water for injection to 2000mL, add 0.1% (g / mL) activated carbon, stir for 10 minutes, and then filter the medicinal solution through a 0.22um filter membrane. The filtrate is divided into vials, placed in a freeze dryer that has been cooled to -20°C, and kept frozen at -20°C for 3 hours, then lowered to about -45°C, vacuumed, and heated within 10 hours To -10°C, the time to maint...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com