Wrapped tablets dichlororeytadin and its preparing method
A technology of desloratadine and coated tablets, which is applied in the field of pharmaceutical compositions and preparations thereof, can solve the problems of desloratadine being unstable when exposed to oxygen and moisture, and degraded by oxygen, so as to solve the problems of moisture degradation, Improve stability, reduce the effect of handlers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
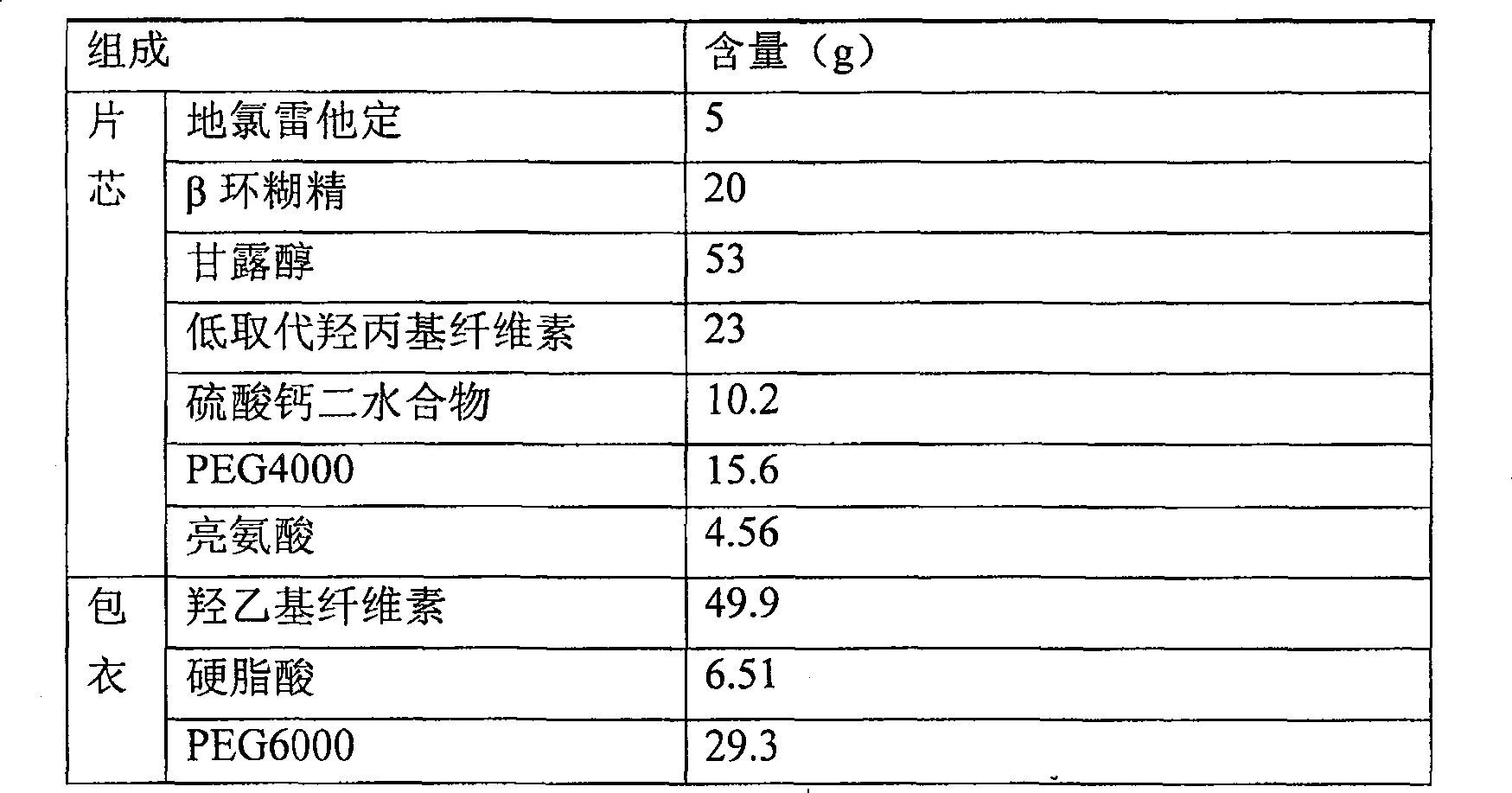
[0038] Prescription and preparation method of coated tablet with 2.3% desloratadine content
[0039]
[0040] 1) At a temperature below 20°C, after grinding and mixing 5g of desloratadine, 20g of β-cyclodextrin and 15.6g of PEG4000 through a ball mill, the resulting mixture was granulated and pulverized with a 1.0mm screen of a rapid granulator to obtain granules;
[0041] 2) Mix the granules obtained in operation 1) with 53g of mannitol, 23g of low-substituted hydroxypropyl cellulose, 10.2g of calcium sulfate dihydrate and 4.56g of leucine, and then press it to obtain a tablet core. The hardness of the tablet is controlled at 3.2 kg / cm2 to 4.5kg / cm2;
[0042] 3) Mix 49.9g of hydroxyethyl cellulose, 6.51g of stearic acid and 29.3g of PEG6000 evenly to obtain a coating material;
[0043] 4) Take the coating material (accounting for 3% of the total tablet weight) and fill it on the die of the tablet press, then add the tablet core, then fill the coating material (accounting fo...
Embodiment 2
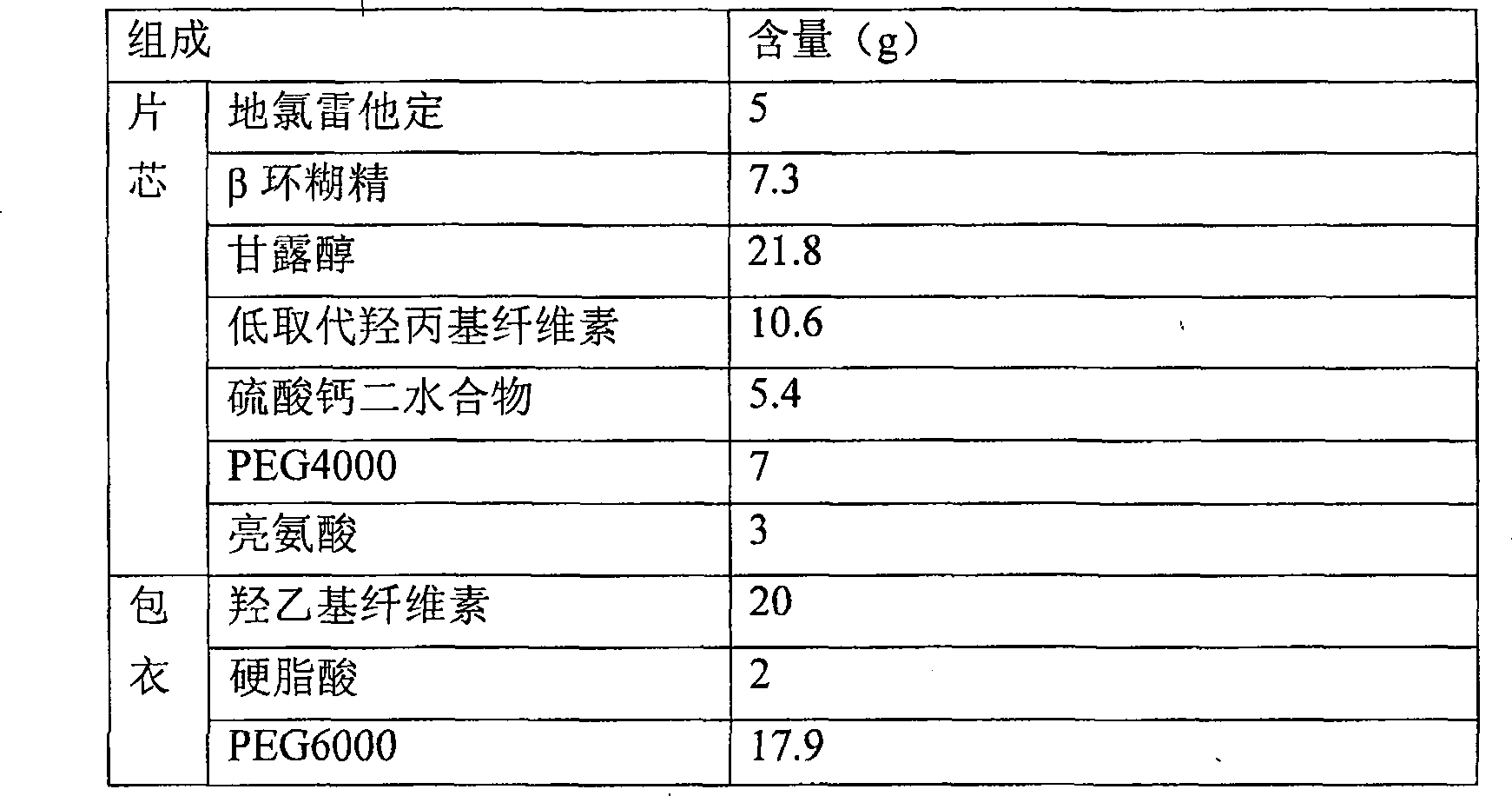
[0045] Prescription and preparation method of coated tablet with 5.0% desloratadine content
[0046]
[0047] 1) At a temperature below 20°C, 5g of desloratadine, 7.3g of β-cyclodextrin and 7g of PEG4000 are mixed uniformly by a ball mill, and the resulting mixture is granulated and crushed with a 1.0mm sieve of a rapid granulator to obtain granules;
[0048] 2) Mix the granules obtained in operation 1) with 21.8g of mannitol, 10.6g of low-substituted hydroxypropyl cellulose, 5.4g of calcium sulfate dihydrate and 3g of leucine, and then compress to obtain a tablet core, and control the hardness of the tablet at 3.2kg / cm2 to 4.5kg / cm2;
[0049] 3) Mix 20g of hydroxyethyl cellulose, 2g of stearic acid and 17.9g of PEG6000 evenly to obtain a coating material;
[0050] 4) Take the coating material (accounting for 3% of the total tablet weight) and fill it on the die of the tablet press, then add the tablet core, then fill the coating material (accounting for 3% of the total ta...
Embodiment 3
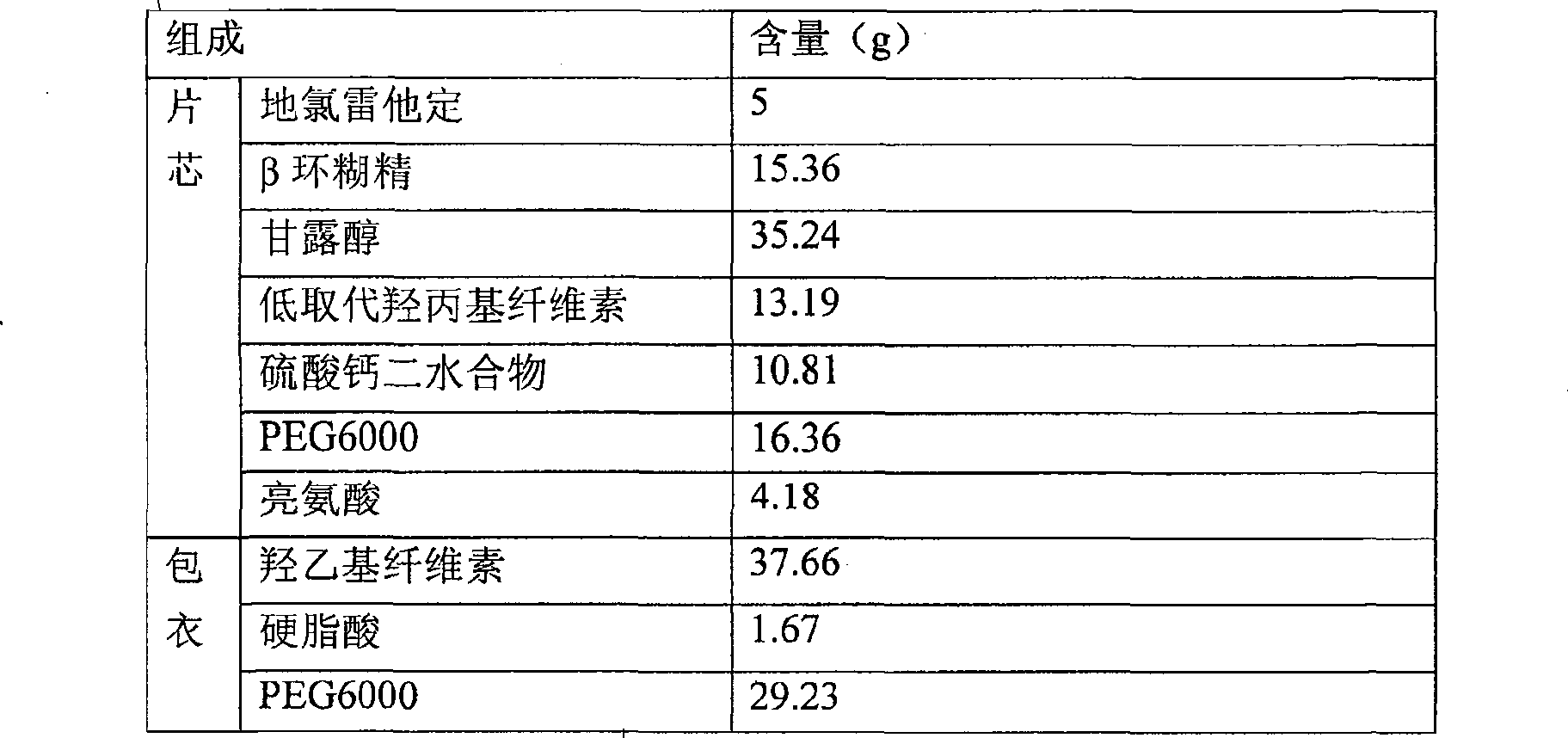
[0052] Prescription and preparation method of coated tablet with 3.0% desloratadine content
[0053]
[0054] 1) At a temperature below 20°C, after grinding and mixing 5g of desloratadine, 15.36g of β-cyclodextrin and 16.36g of PEG6000 by a ball mill, the resulting mixture was granulated and pulverized with a 1.0mm screen of a rapid granulator to obtain granules;
[0055] 2) Mix the granules obtained in operation 1) with 35.24g of mannitol, 13.19g of low-substituted hydroxypropyl cellulose, 10.81g of calcium sulfate dihydrate and 4.18g of leucine, and then press it into a tablet core to control the hardness of the tablet At 3.2kg / cm2 to 4.5kg / cm2;
[0056] 3) Mix 37.66g of hydroxyethyl cellulose, 1.67g of stearic acid and 29.23g of PEG6000 evenly to obtain a coating material;
[0057] 4) Take the coating material (accounting for 3% of the total tablet weight) and fill it on the die of the tablet press, then add the tablet core, then fill the coating material (accounting fo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com