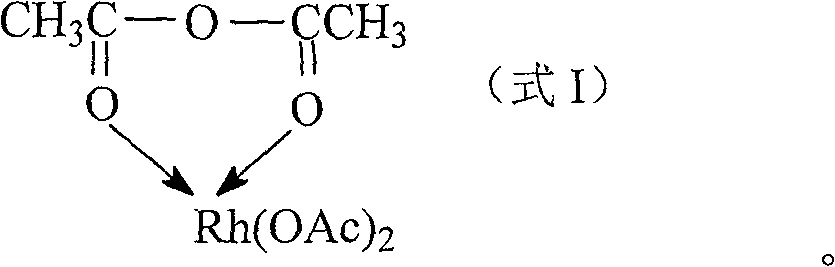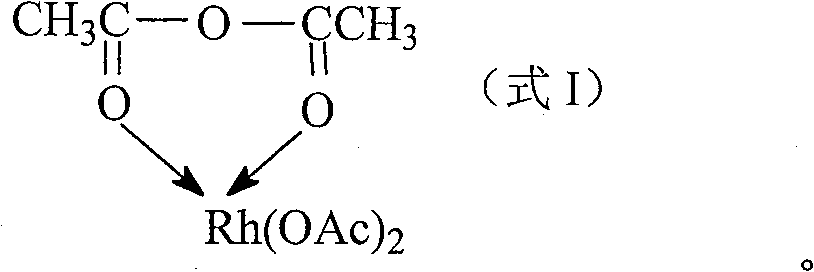Rhodium acetic anhydride complex, and its preparing method and application
A technology of rhodium acetic anhydride and complexes, applied in the field of acetic anhydride rhodium complexes and its preparation, can solve problems such as poor catalyst stability, limited theoretical research, and no value for industrial development, and achieve excellent catalytic activity and selectivity The effect of improving the performance and catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Weigh 0.6 mole of acetic anhydride and dissolve it in 3.0 mole of methanol, stir well and divide into three parts. Under stirring in an ice bath, 0.1, 0.2, 0.3 moles of rhodium acetate were added respectively. After continuing to stir for 20 minutes, add excess diethyl ether to precipitate and filter; the product was washed with diethyl ether three times, and depressurized at room temperature to constant weight to obtain acetic anhydride rhodium complex.
[0019] The elemental analysis results of the complex are as follows:
[0020] the element
Embodiment 2
[0022] Add 0.2 grams of acetic anhydride rhodium complex, 2.48 moles of methanol, 0.65 moles of acetic acid, and 0.34 moles of methyl iodide in the reactor. After feeding CO, the temperature is raised to 140 ° C, the reaction pressure is kept at 4.0 MPa, and the stirring speed is 500 rpm. Time 30 minutes. After the reaction, the product composition was determined by gas chromatography: the conversion rate of methanol was 100%, and the product composition was 0.82 moles of methyl acetate and 1.45 moles of acetic acid.
Embodiment 3
[0024] Add 0.25 grams of rhodium complexes, 2.48 moles of methanol, 0.5 moles of acetic acid, 0.34 moles of methyl iodide, and 0.08 moles of lithium iodide in the reaction kettle. After feeding CO, the temperature was raised to 150° C., the pressure was maintained at 4.0 MPa, the stirring speed was 500 rpm, and the reaction time was 20 minutes. After the reaction, the product composition was determined by gas chromatography: the conversion rate of methanol was 100%, and the product composition was 0.57 moles of methyl acetate and 1.80 moles of acetic acid.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com