IIIV vaccine containing recombination virus and its united immune preparation
A technology for immune preparations and recombinant adenoviruses, applied in antiviral agents, biochemical equipment and methods, applications, etc., can solve the problems of virus genetic background and activity influence, adenovirus genome prone to mutation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Preparation of the transformed gag gene (mod.gag) of embodiment 1
[0065] In this example, through codon optimization, a modified gag gene (mod.gag) capable of expressing in eukaryotic cells independent of the regulatory protein Rev was obtained.
[0066] 1.1 Cloning of HIV-1 gag gene and acquisition of consensus sequence
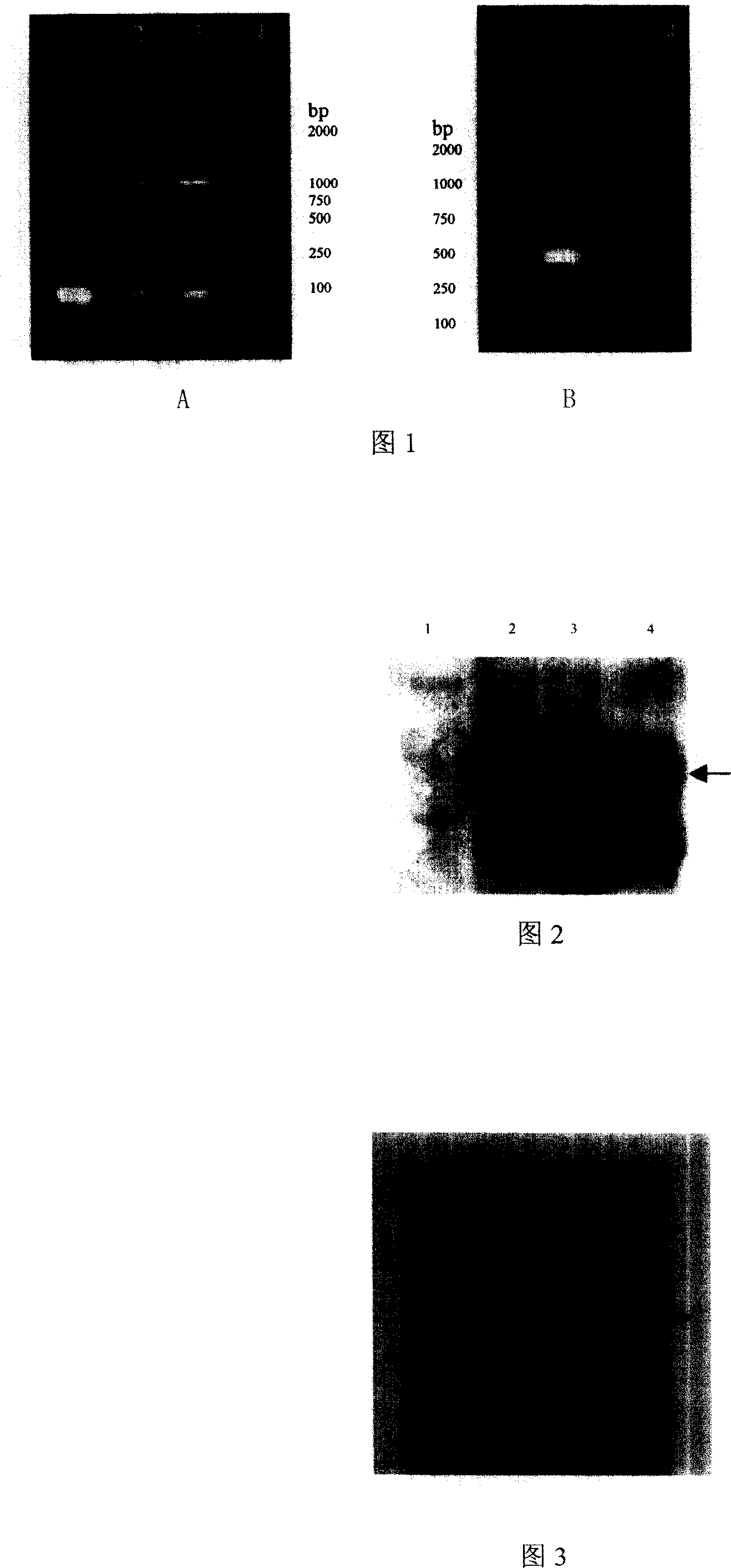
[0067] Collect 20 samples of venous blood from HIV-1 antibody-positive patients in HIV-1 endemic areas in Henan, 5ml each, anticoagulate with heparin, use Qiagen’s QiaAmp Blood reagent, extract cellular DNA according to the instructions, and store nucleic acid samples at -20°C. The gag gene was amplified by nested-PCR method. Use G-1 / G-2 (G-1: 5'CGA CGC AGG ACT CGG CTT GC 3'; G-2: 5'CCT GGCTTT AAT TTT AC 3') as the outer primers for the first PCR reaction, The conditions are: pre-denaturation at 94°C for 5 min, 30 cycles at 94°C for 45 sec, 55°C for 45 sec, and 72°C for 210 sec; 72°C for 10 min. Take one tenth of the PCR product and use G-3 / G-4 (...
Embodiment 2
[0071] Construction and identification of the recombinant virus of embodiment 2 containing mod.gag gene
[0072] 2.1 Construction and passage of replication-deficient recombinant adenovirus containing mod.gag gene
[0073] 2.1.1 Construction of recombinant adenovirus
[0074] Using the plasmid pUC57-mod.gag as a template, primers hn-a / hn-2 (hn-a: 5'CGG AAT TCT ACC GCC ACCAT 3'; hn-2: 5'GCG TCG ACT TAT TGT GAC GAG GGG T 3') Perform PCR reaction for the upstream and downstream primers respectively, the conditions are: 94°C 5min pre-denaturation; 94°C 45sec, 55°C 45sec, 72°C 105sec, 30 cycles; 72°C 10min. The PCR product was separated by 1% agarose gel electrophoresis, and the 1.5 kb size-specific amplification band was recovered after being judged to be correct with the control. The recovered PCR product and the shuttle plasmid pDC316 (purchased from Canada Microbix Biosystems, Inc. company), respectively recovered the former 1.5kb mod.gag gene fragment and the latter 3.9kb pD...
Embodiment 3
[0093] Example 3 Recombinant virus immune effect test
[0094] 3.1 Mouse immunization experiment of recombinant adenovirus
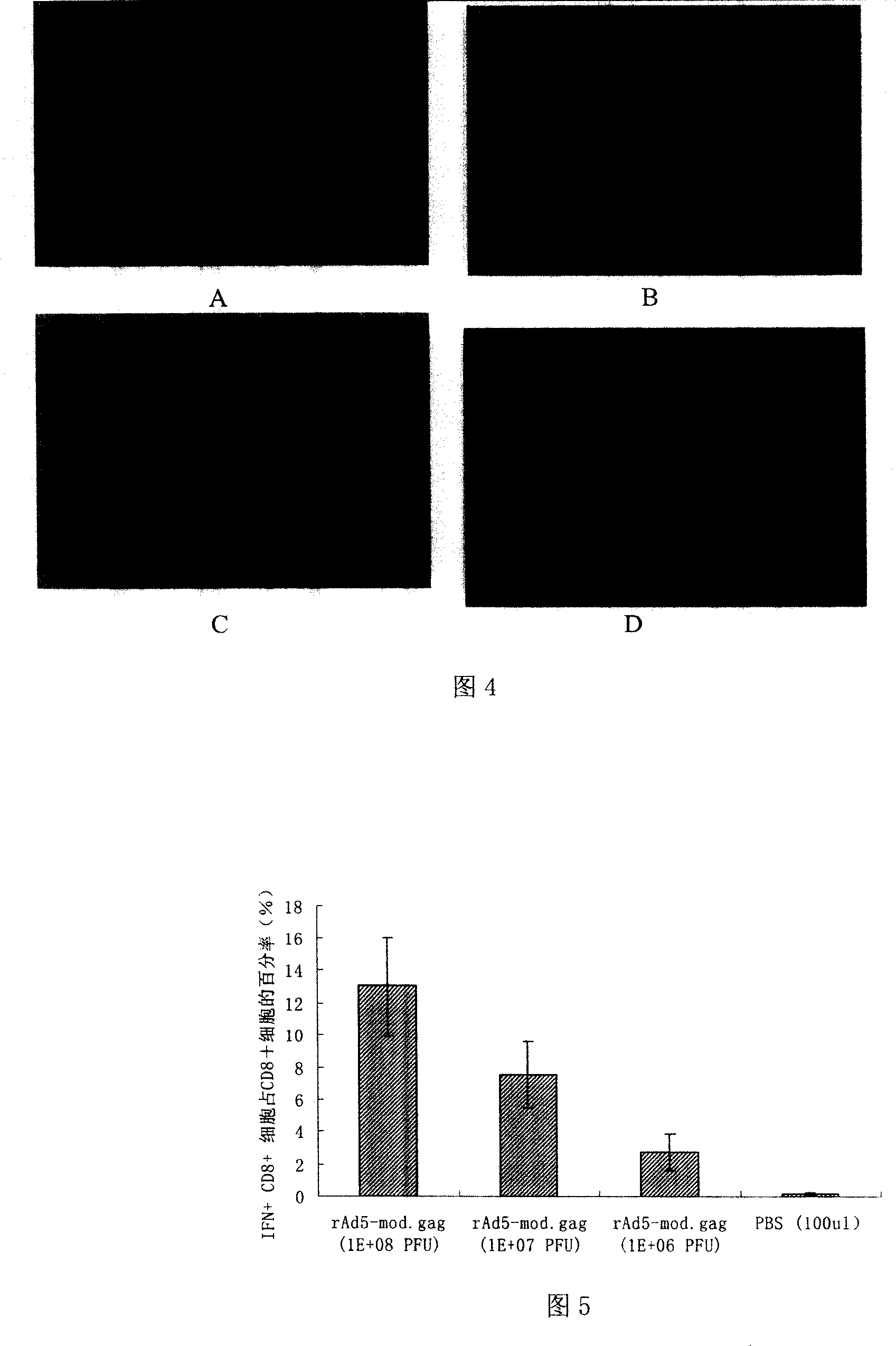
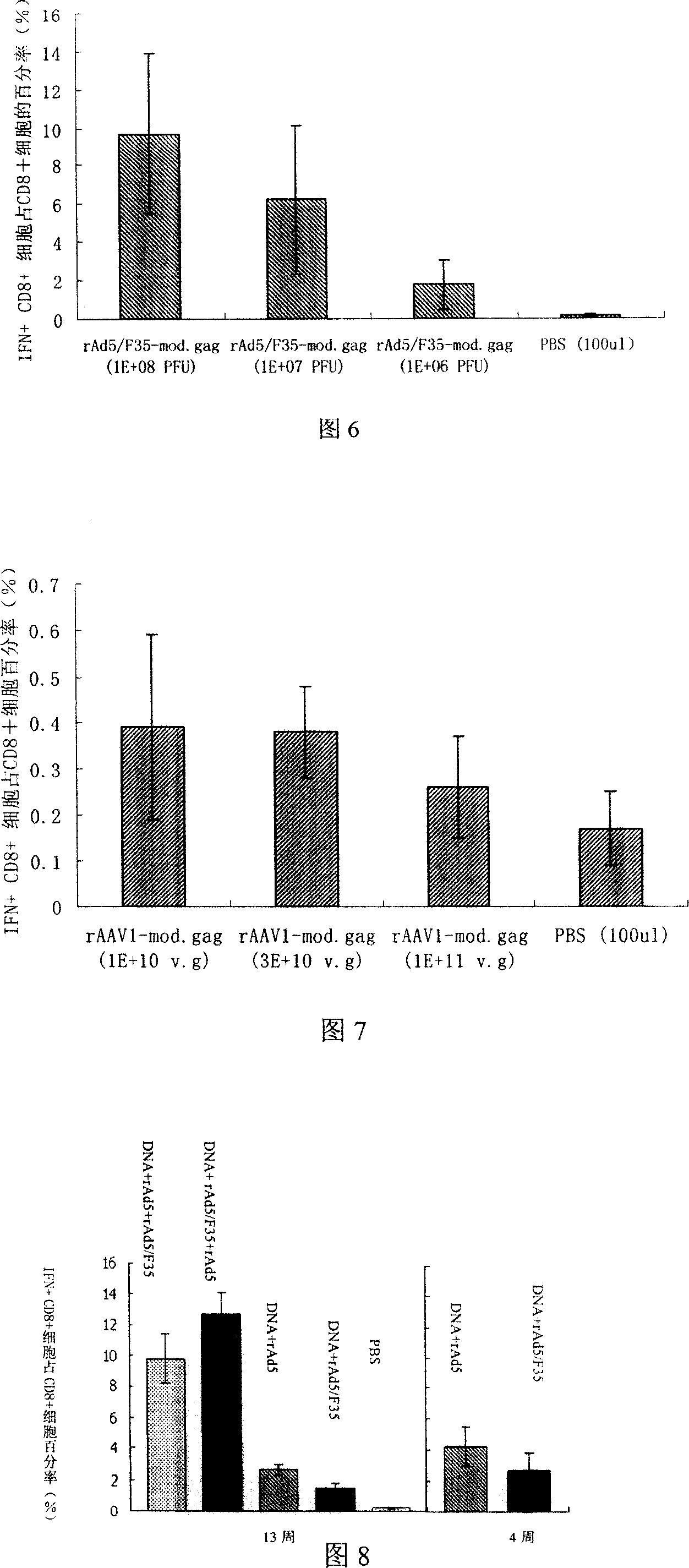
[0095] Forty female BALB / c mice, 4-6 weeks old, weighing about 18-25 grams, were randomly divided into 8 groups, 5 mice in each group. 4 groups were used to detect the immune effect of rAd5-mod.gag in mice, and the other 4 groups were used to detect the immune effect of rAd5 / F35-mod.gag in mice. The two recombinant viruses used the same dose. Individual immunizations were performed with different doses of rAd5-mod.gag or rAd5 / F35-mod.gag vaccines. Dilute the two recombinant virus vaccines to 10 7 PFU / ml, 10 8 PFU / ml and 10 9 PFU / ml, unilateral tibialis anterior muscle injection, 100 μl each time, and the control group was injected with the same volume of sterile PBS. See Table 1 for specific immunization procedures and doses.
[0096] Table 1. Individual immunization schedules of different doses of recombinant adenovirus vaccines
[0097] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com