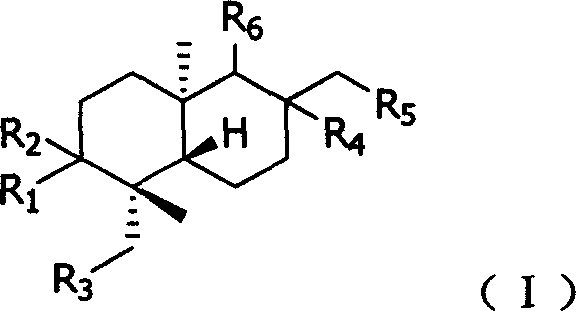
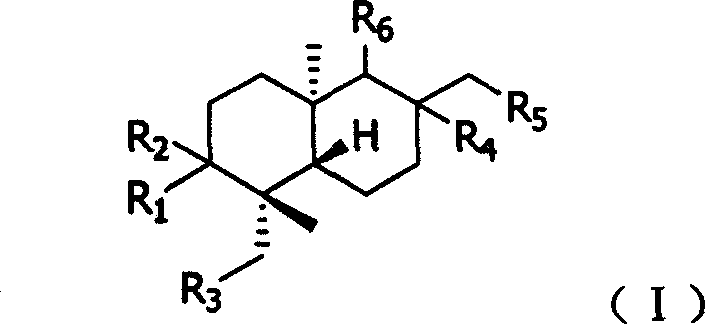
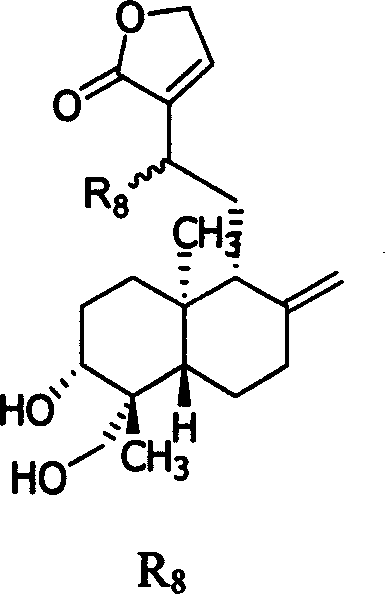
Substituted andrographolide derivative, preparing method and pharmaceutical compound thereof
An unsubstituted and compound technology, which is applied in the field of substituted andrographolide derivatives, can solve problems such as no reports on the activity of andrographolide derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0113] 3, the preparation of 19-isopropylidene andrographolide (2):
[0114] In a 500ml round bottom flask, add 15.0g andrographolide (1) (42.9mmol), 20ml2,2-dimethoxypropane, catalytic amount of pyridinium p-toluenesulfonate (PPTs), 300ml benzene, 40ml DMSO, heat to reflux 30min, the reaction is complete. Cool the reaction solution to room temperature, add triethylamine (about 10ml) to adjust the pH to 8, add 200ml benzene to dilute the reaction solution, wash with saturated brine (300ml×3), dry over anhydrous sodium sulfate, filter and concentrate, solids are precipitated, filter 15.0 g of white solid was obtained, yield 90%, mp: 194-196° C. (documentation mp: 194.5° C.).
Embodiment 2
[0116] 3, the preparation of 19-isopropylidene-12-hydroxyl-andrographolide (3):
[0117] Add 4.0g2 (10.3mmol), 5.0gPDC (13.3mmol), and 60ml of dichloromethane into a 100ml round bottom flask, heat to reflux, and monitor the reaction by TLC. After the reaction was completed, the heating was stopped, cooled to room temperature, and separated by column chromatography (petroleum ether: ethyl acetate = 3:2) to obtain 2.8 g of a white solid, with a yield of 70%.
Embodiment 3
[0119] 12-propionyloxy-14-deoxyandrographolide (IA 1 ) preparation:
[0120] Add 1.0g3 (2.6mmol), 2ml propionic anhydride (21.2mol), catalytic amount of DMAP, 25ml dichloromethane into a 50ml round bottom flask, react at 25°C for 12h, evaporate the solvent under reduced pressure, and dissolve the residue with 150ml ethyl acetate , washed with saturated brine, dried over anhydrous sodium sulfate, and evaporated to obtain light-colored oil 4. Add 7ml of acetic acid, 3ml of water, and react at 25°C for 1h. The reaction solution was dissolved in 100ml of ethyl acetate, saturated saline, and saturated carbonic acid Sodium hydrogen solution was washed to pH about 7, dried over anhydrous sodium sulfate, and the solvent was evaporated to obtain a light-colored oil, which was separated by column chromatography (petroleum ether: ethyl acetate = 3:2) to obtain 0.63 g of a white solid. Rate 60%, mp: 141-142°C.
[0121] IR (KBr) v: 3342, 3133, 2939, 2864, 1754, 1730, 1646, 1446, 1341, 11...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com