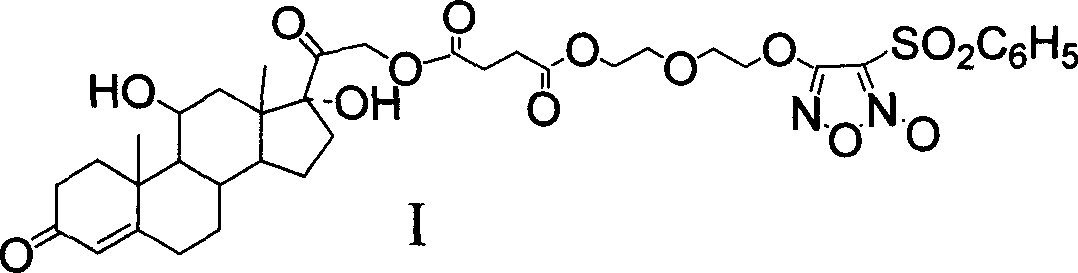
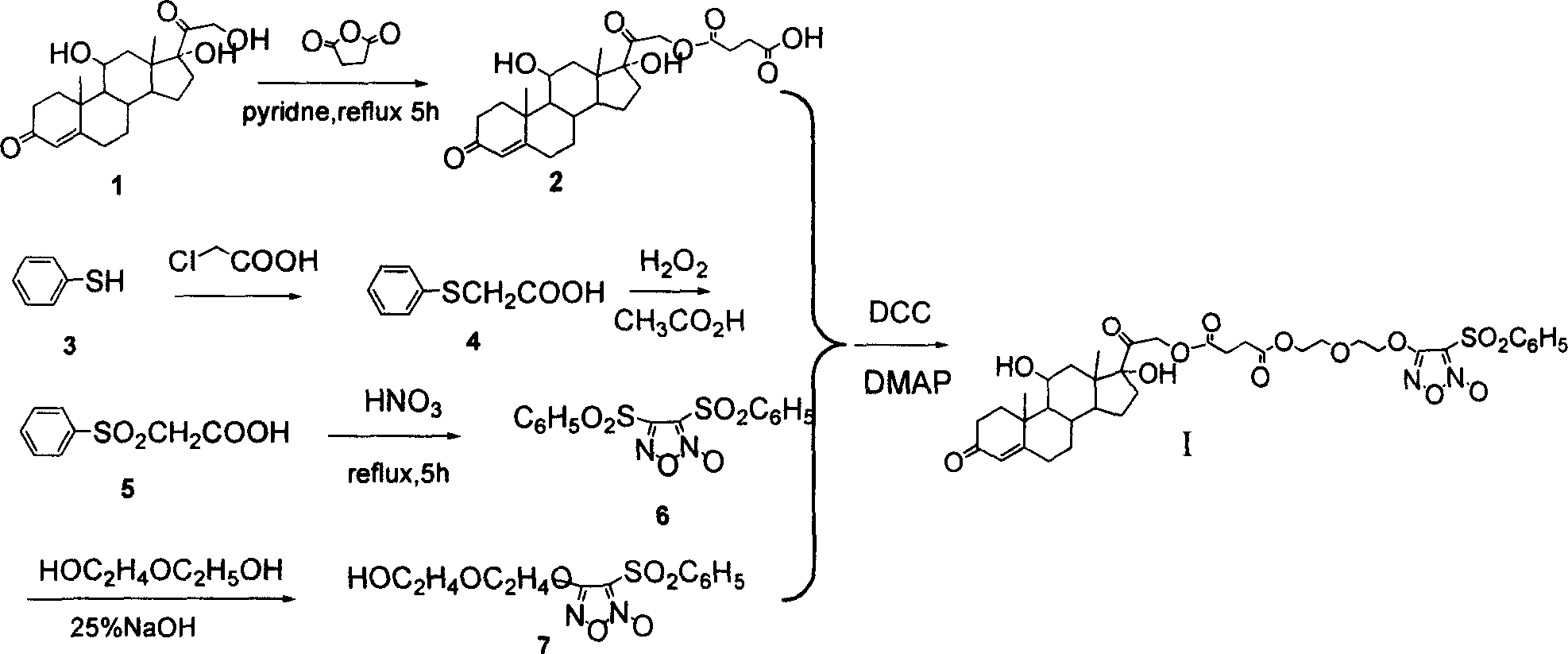
NO donor-type hydrocortisone derivative, its preparing method and antiphlogistic use
A technology for hydrocortisone and uses, which is applied in the direction of anti-inflammatory agents, steroids, and medical preparations containing active ingredients, which can solve problems such as inducing osteoporosis, achieve overcoming side effects, good therapeutic effects, and excellent anti-inflammatory effects. The effect of inflammatory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Synthesis of 4-(hydrocortisone-21-oxo-)-4-oxo-butyric acid (2)
[0053] Get a 100ml eggplant-shaped bottle, dissolve 5.43g (15mmol) hydrocortisone (1) in 75ml pyridine, add 3g (30mmol) succinic anhydride, heat and reflux for 8 hours to stop the reaction, pour into 100ml ice water after cooling, 5% HCl was used to adjust the pH to 5.0-5.5, and a white solid was precipitated, which was filtered and washed with water to obtain 5.95 g of the product, with a yield of 86%. IR (v, cm -1 ): 3428vs, 1730vs, 1711vs; ESI-MS: [M+H] + = 463; 1 H-NMR (300MHz, CDCl 3 ): δ=0.96[s, 3H, C(19)-CH 3 ], 1.00-1.05[m, 1H, C(9)-H], 1.10-1.26[m, 2H, C(6)-H b , C(15)-H b ], 1.44[s, 3H, C(18)-CH 3 ], 1.47-1.61 [m, 4H, C(12)-H b , C(14)-H, C(15)-H a , C(16)-H b ], 1.66-1.81 [m, 2H, C(12)-H a , C(1)-H b ], 1.83-1.95[m, 3H, C(1)-H a , C(1)-H, C(6)-H a ], 1.91-2.15[m, 4H, C(7)-H, C(2)-H b , C(2)-H a , C(16)-H a ], 2.16-2.27[m, 2H, C(5)-H a , C(5)-H b ], 2.27-2.39 [m, 2H, C(24)-CH ...
Embodiment 2
[0063] Mix 50 mg of Compound I of the present invention with 100 mg of starch and 100 mg of lactose, use an appropriate amount of 5% polyvinylpyrrolidone as a wetting agent to make a soft material, granulate in a conventional manner, dry, add 3 mg of magnesium stearate and mix to make a tablet.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com