Targeting chitosan carrier guided by folic acid receptor and the preparing method and the application
A technology of folic acid receptor and chitosan, applied in medical preparations with non-active ingredients, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve myocardial cell damage, doxorubicin limitation, strong cardiotoxicity and other issues, to achieve the effect of high bioavailability, obvious targeting, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] 0.2g of chitosan with a molecular weight of 200kDa and a degree of deacetylation of 92% was dissolved in 20ml of HAC-NaAC buffer at pH=4.7;
[0058] Dissolve 0.0044g of folic acid in 3ml of anhydrous DMSO (dimethyl sulfoxide), add 0.0019g of EDC·HCl, and stir at 25°C for 1 hour to activate the carboxyl group of folic acid;
[0059] The above-mentioned chitosan solution was mixed with folic acid solution, and stirred and reacted in the dark for 15 hours, and the pH value of the mixed solution after the reaction was adjusted to 9.0 with 5mol / L aqueous sodium hydroxide solution, and the mixture was placed in the phosphate buffer solution of pH=7.4 Dialyzed against medium for 3 days, then dialyzed in deionized water for 3 days, freeze-dried in vacuum to obtain chitosan-folate complex.
[0060] The general structural formula of the obtained chitosan-folate complex is as formula 1, wherein: n=1150;
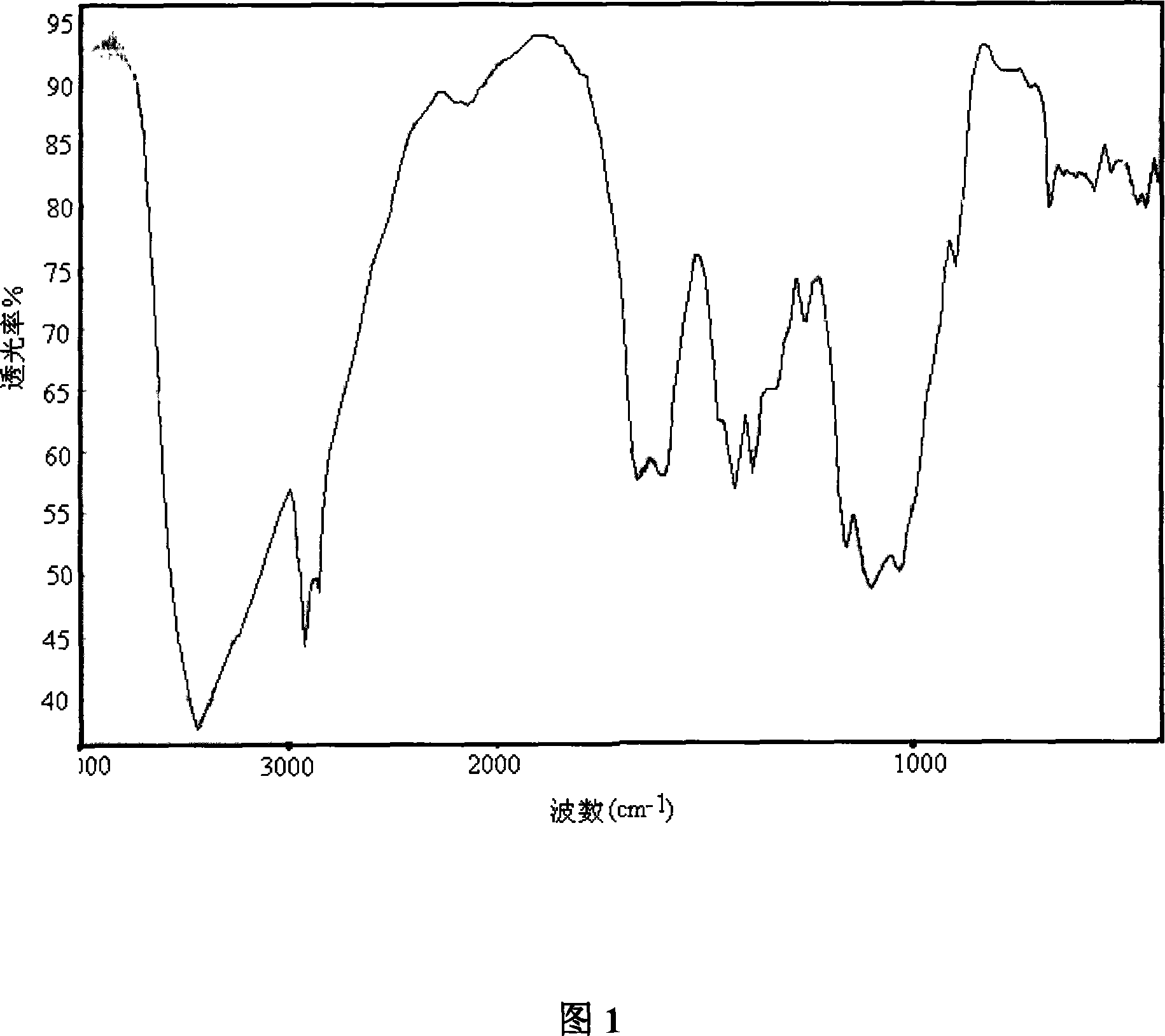
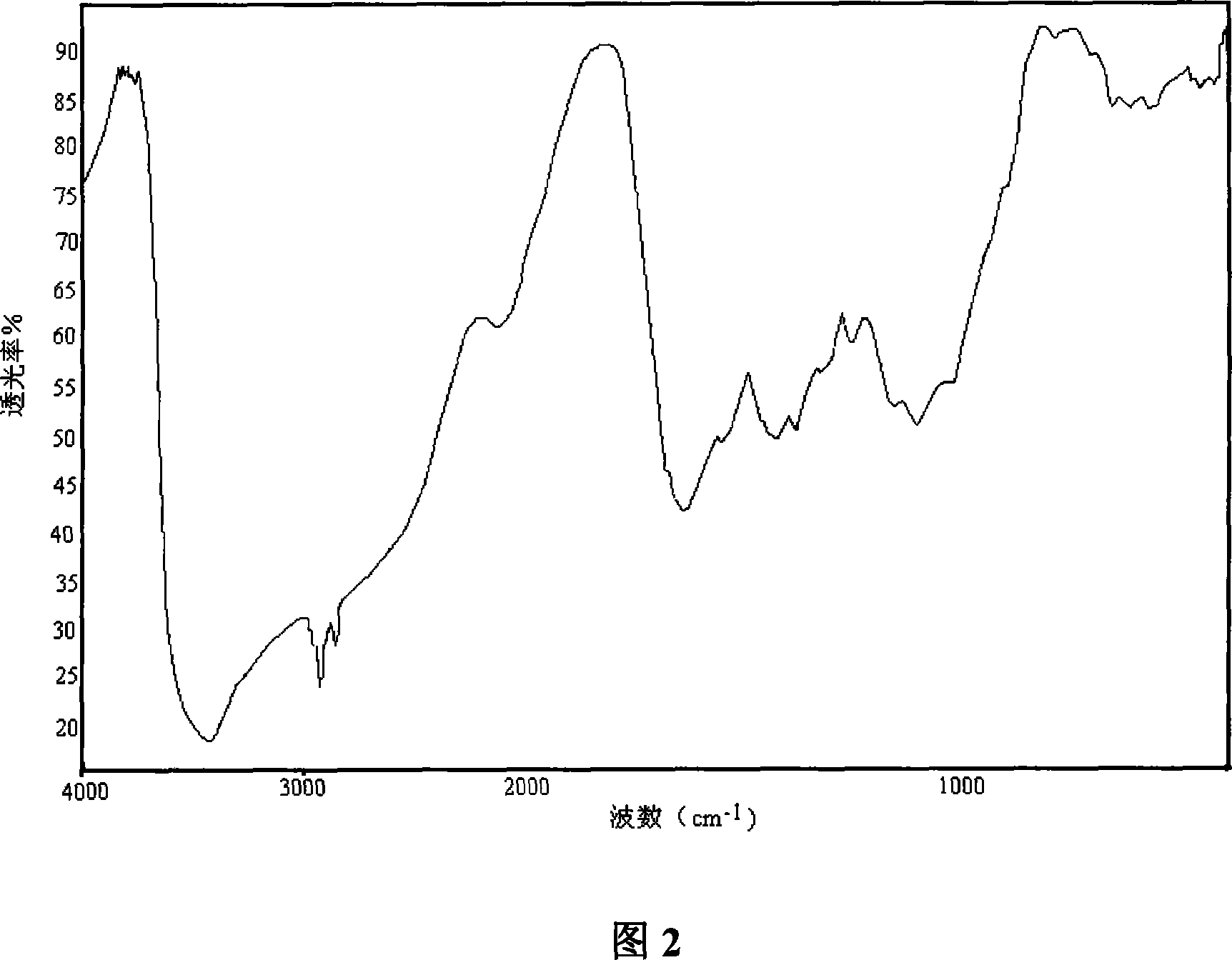
[0061] The infrared spectrum of chitosan is shown in Figure 1; the infrared...
Embodiment 2
[0067] 0.2g of chitosan with a molecular weight of 200kDa and a degree of deacetylation of 92% was dissolved in 20ml of HAC-NaAC buffer at pH=4.7;
[0068] Dissolve 0.0044g of folic acid in 3ml of anhydrous DMSO, add 0.0019g of EDC·HCl, and stir at 25°C for 1 hour to activate the carboxyl group of folic acid;
[0069] The above-mentioned chitosan solution was mixed with folic acid solution, and stirred and reacted in the dark for 15 hours, and the pH value of the mixed solution after the reaction was adjusted to 9.0 with 5mol / L aqueous sodium hydroxide solution, and the mixture was placed in the phosphate buffer solution of pH=7.4 Dialyzed against medium for 3 days, then dialyzed in deionized water for 3 days, freeze-dried in vacuum to obtain chitosan-folate complex.
[0070] The general structural formula of the obtained chitosan-folate complex is as formula 1, wherein: n=1150;
[0071] The identification of chitosan-folate complex is the same as in Example 1.
[0072]Disso...
Embodiment 3
[0076] 0.2g of chitosan with a molecular weight of 200kDa and a degree of deacetylation of 92% was dissolved in 20ml of HAC-NaAC buffer at pH=5.5;
[0077] Dissolve 0.0044g of folic acid in 3ml of anhydrous DMSO, add 0.0019g of EDC·HCl, and stir at 25°C for 1 hour to activate the carboxyl group of folic acid;
[0078] The above-mentioned chitosan solution was mixed with folic acid solution, and stirred and reacted in the dark for 20 hours, and the pH value of the mixed solution after the reaction was adjusted to 9.0 with 5mol / L sodium hydroxide aqueous solution, and the mixture was placed in the phosphate buffer solution of pH=7.4 Dialyzed against medium for 3 days, then dialyzed in deionized water for 3 days, freeze-dried in vacuum to obtain chitosan-folate complex.
[0079] The general structural formula of the obtained chitosan-folate complex is as formula 1, wherein: n=1150;
[0080] The identification of chitosan-folate complex is the same as in Example 1.
[0081] 0.00...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
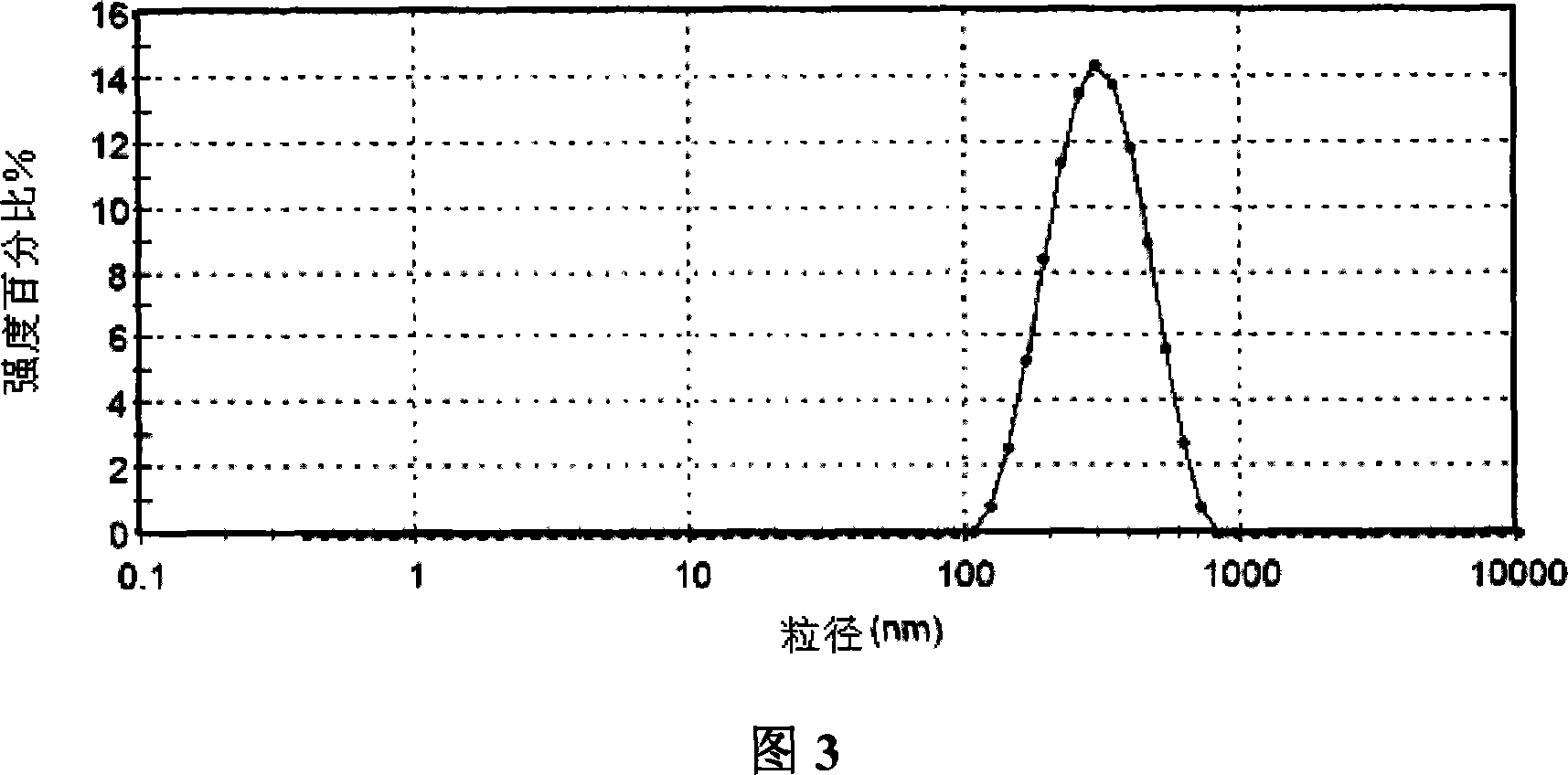
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com