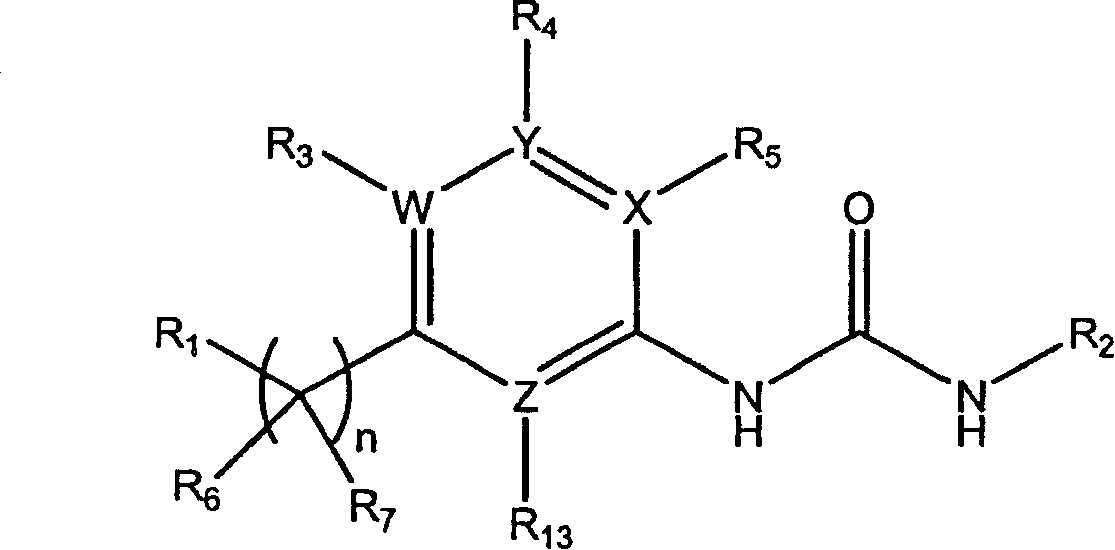
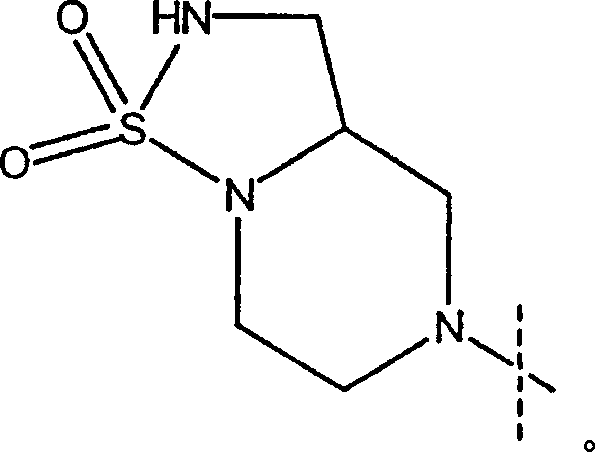
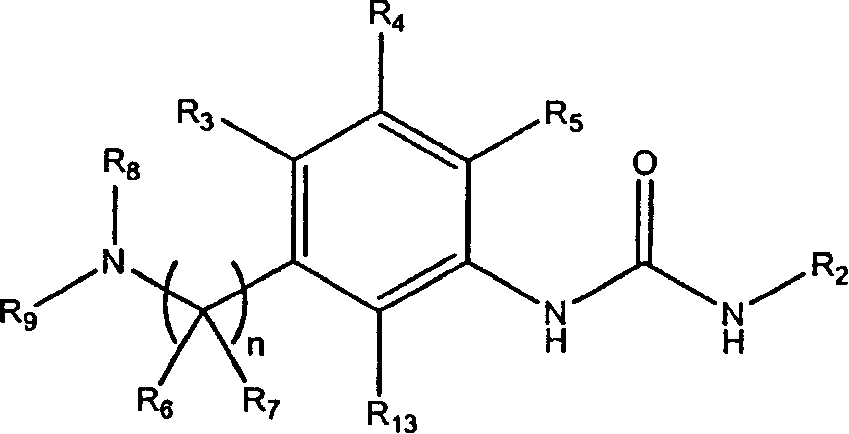
Substituted urea derivatives for treating cardiac diseases
A compound, chelate technology, applied in the field of pharmaceutical compositions, chemical entities for the treatment of heart disease, can solve problems such as unfavorable heart failure patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0793] Example 1 Step 1
[0794]
[0795] To 1.0 equivalent of 1A anhydrous DMF (0.37M) solution, add Zn(CN) 2 (0.92 equivalent) and Pd (PPh 3 ) 4 (0.058 equivalent). Nitrogen was introduced into the reaction mixture and heated to 80°C overnight. Then add another 0.023 equivalent of Pd(PPh 3 ) 4 , And heat the reaction for another 6 hours. Then the reaction mixture was cooled to RT, diluted with 15 times volume of EtOAc (calculated as 1A), and the organic layer was washed 3 times with water and once with saline solution. The organic layer was dried over sodium sulfate, filtered and concentrated. Chromatographic purification on silica gel, with 10% Et 2 O / hexane was the eluent, and 1B (90%) was obtained as a solid.
[0796] Example 1 Step 2
[0797]
[0798] Less than 1.0 equivalent of 1B anhydrous Et 2 To the O (0.06M) solution, the diisobutyllithium aluminum hydride solution (1.1 equivalent, 1.0M solution in hexane) was added dropwise with a syringe. The resulting solution wa...
Embodiment 2
[0813] Example 2 Step 1
[0814]
[0815] To 1.0 equivalent of (4-fluoro-3-nitro-phenyl)-methanol (2A) in THF (about 1M 2A in THF) and (about 1.1 equivalents) of pyridine, about 1.1 equivalents of methanesulfonyl chloride was added. The mixture was stirred at room temperature overnight and then concentrated. The residue was purified by flash chromatography on silica gel with 10%-50% EtOAc / hexane as the eluent to obtain 4-fluoro-3-nitro-benzyl methanesulfonate (2B) (57%).
[0816] Example 2 Step 2
[0817]
[0818] To 1.0 equivalent of 4-fluoro-3-nitro-benzyl methanesulfonic acid (2B) in DMF solution (about 0.6M 2B DMF solution), add about 1.05 equivalent of TEA and about 1.0 equivalent of tert-butyl piperazine-1-carboxylate ester. The mixture was stirred at room temperature for 30 minutes, diluted with EtOAc, and NH 4 Cl solution washing, drying (Na 2 SO 4 ),evaporation. Purified by flash chromatography on silica gel using 50% EtOAc / hexane as the eluent to obtain 4-(4-fluoro-3...
Embodiment 3
[0826] Example 3 Step 1
[0827]
[0828] At RT and N 2 Next, fill a round bottom flask with 1 equivalent of 3-chloro-2-fluoroaniline (3A), 1-methyl-2-pyrrolidone (about 1.5M 3A in NMP solution), 2.2 equivalents of sodium cyanide, and 1.35 equivalents of bromine Nickel(II). To N 2 At the same time, introduce additional NMP to reduce the concentration by half, and slowly warm the solution to 200±5℃, 2 After stirring for 4 days. The reaction mixture was allowed to cool to room temperature. The reaction mixture was diluted with 30 times the volume of tert-butyl methyl ether (MTBE) and filtered through Celite. Then wash the diatomaceous earth pad with 10 times the volume of MTBE. Organic matter is washed with 40 times volume of salt solution, 2×40 times volume of water and 40 times volume of salt solution. The combined organics were dried over sodium sulfate and concentrated to obtain a brown solid, which was vacuum dried at 40°C (~30 inches Hg) for 8 hours to obtain the compound of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com