Application of salvia miltiorrhiza bge I sodium sulfonate in the preparation of medicine and its medicine made by the same
A technology of sodium sulfonate and tanshinone, applied in the application of tanshinone 1 sodium sulfonate in pharmacy, and the pharmaceutical field with tanshinone 1 sodium sulfonate as a component, can solve problems such as tanshinone 1 sodium sulfonate not being effectively utilized and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
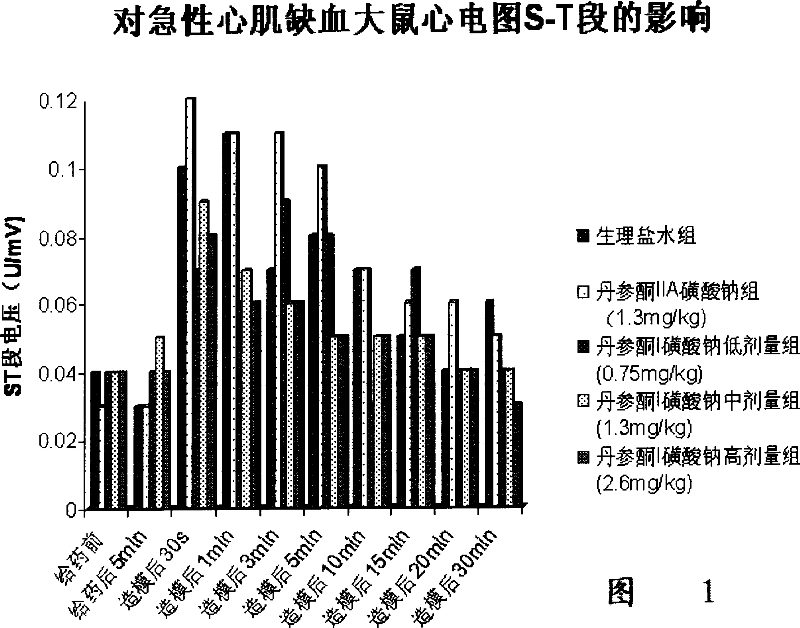
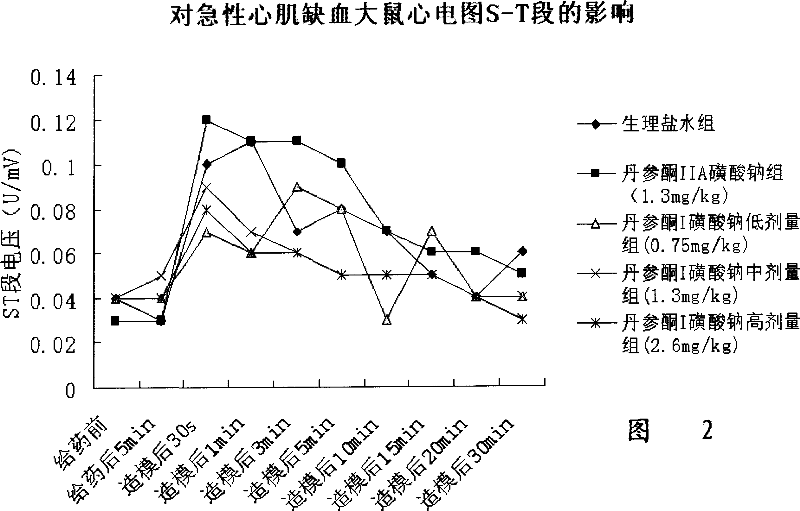
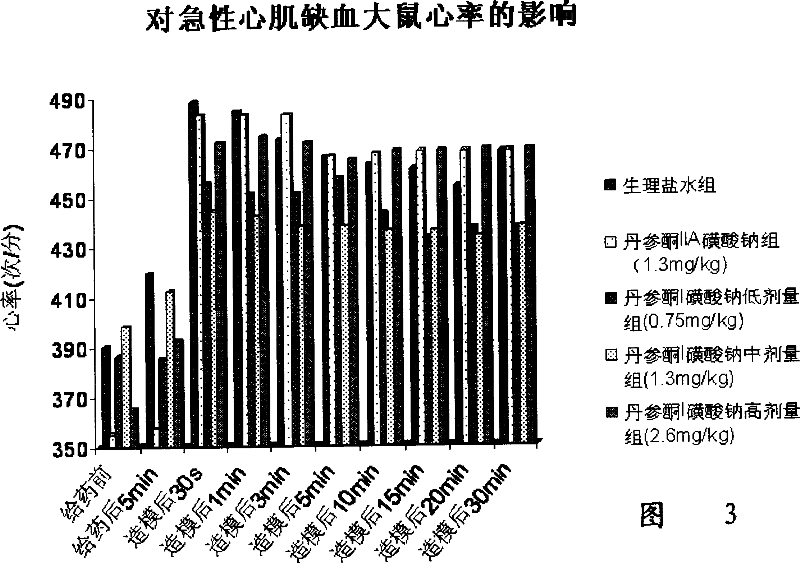
Image
Examples
Embodiment 1
[0129] Synthesis and Structure Confirmation of Sodium Tanshinone I Sulfonate
[0130] 1) Synthesis: Get 0.2 gram of Tanshinone I and drop into a three-necked bottle filled with 5.2ml of glacial acetic acid and 7.2ml of acetic anhydride, add dropwise 0.4ml of concentrated sulfuric acid-glacial acetic acid (V:V=1:1) under ice-water bath, Stir at room temperature for 4 hours, slowly add 20ml of saturated NaCl solution, stir for 0.5 hours, filter with suction, wash the filter cake with saturated NaCl solution (10ml, twice), wash the filter cake with water, make the filter cake ) and dried under reduced pressure. Take the filter cake and add 200ml of ethyl acetate to reflux for 6 hours, remove unsulfonated fat-soluble impurities, filter, take the solid matter and dissolve it in 400ml of absolute ethanol, reflux for 4 hours, filter, take the filtrate, remove the solvent under reduced pressure, and obtain reddish-brown The solid was recrystallized from methanol to obtain the target ...
Embodiment 2
[0136] Synthesis and Structure Confirmation of Sodium Tanshinone I Sulfonate
[0137] 1), Synthesis: Take 5g of Tanshinone I raw material, add 13ml of glacial acetic acid, then add 20ml of acetic anhydride, place in a three-necked flask, add 10ml of concentrated sulfuric acid-glacial acetic acid (1:1) dropwise at 10-15°C under stirring After the dropwise addition, stir at room temperature for 1 hour, then slowly pour the reaction solution into an equal volume of distilled water, and quickly add 100ml of saturated aqueous sodium chloride solution, that is, thick sodium tanshinone I sulfonate is precipitated, centrifuged , the precipitate was washed twice with saturated sodium chloride solution, and then washed once with an appropriate amount of water to make the solid matter pH=5-6, and the solid matter was evaporated to dryness on a water bath to obtain 6.1 g of brown-red sodium tanshinone Isulfonate crude product , Yield: 80.8%. Dissolve the crude sodium tanshinone I sulfona...
Embodiment 3
[0142] Preparation of Sodium Tanshinone I Sulfonate Lyophilized Powder for Injection
[0143] Take 4g of sodium tanshinone I sulfonate and 40g of mannitol, add water for injection to 2000ml (1000 counts), stir and heat to 60°C to dissolve, use 0.2% (w / v) activated carbon for needles, decarbonize by coarse filtration, and use 0.22um microporous membrane fine filtration, detection of intermediate content, filling 2ml / bottle, semi-pressed butyl rubber stopper, freeze-drying to obtain tanshinone sodium sulfonate freeze-dried powder injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com